Determination of critical concentration for drug susceptibility testing of Mycobacterium tuberculosis against para-aminosalicylic acid with clinical isolates with thyA, folC and dfrA mutations
- PMID: 36335391
- PMCID: PMC9637297
- DOI: 10.1186/s12941-022-00537-z
Determination of critical concentration for drug susceptibility testing of Mycobacterium tuberculosis against para-aminosalicylic acid with clinical isolates with thyA, folC and dfrA mutations
Abstract
Background & objectives: Accurate determination of antimicrobial resistance profiles is of great importance to formulate optimal regimens against multidrug-resistant tuberculosis (MDR-TB). Although para-aminosalicylic acid (PAS) has been widely used clinically, the reliable testing methods for PAS susceptibility were not established. Herein, we aimed to establish critical test concentration for PAS on the Mycobacterial Growth Indicator Tube (MGIT) 960 in our laboratory settings.
Methods: A total of 102 clinical isolates were included in this study, including 82 wild-type and 20 resistotype isolates. Minimum inhibitory concentration (MIC) was determined by MGIT 960. Whole-genome sequencing was used to identify the mutation patterns potentially conferring PAS resistance. Sequence alignment and structure modelling were carried out to analyze potential drug-resistant mechanism of folC mutant.
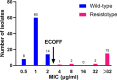
Results: Overall, the Minimum inhibitory concentration (MIC) distribution demonstrated excellent separation between wild-type and resistotype isolates. The wild-type population were all at least 1 dilution below 4 μg/ml, and the resistotype population were no lower than 4 μg/ml, indicating that 4 μg/ml was appropriate critical concentration to separate these two populations. Of 20 mutant isolates, 12 (60.0%) harbored thyA mutations, 2 (10%) had a mutation on upstream of dfrA, and the remaining isolates had folC mutations. Overall, thyA and folC mutations were scattered throughout the whole gene without any one mutation predominating. All mutations within thyA resulted in high-level resistance to PAS (MIC > 32 μg/ml); whereas the MICs of isolates with folC mutations exhibited great diversity, ranged from 4 to > 32 μg/ml, and sequence and structure analysis partially provided the possible reasons for this diversity.
Conclusions: We propose 4 μg/ml as tentative critical concentration for MGIT 960. The major mechanism of PAS resistance is mutations within thyA and folC in MTB isolations. The whole-gene deletion of thyA locus confers high-level resistance to PAS. The diversity of many distinct mutations scattered throughout the full-length folC gene challenges the PCR-based mutation analysis for PAS susceptibility.
Keywords: Critical Concentration; Drug Susceptibility Testing; Mycobacterium tuberculosis; Para-Aminosalicylic Acid.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Molecular genetics of para-aminosalicylic acid resistance in clinical isolates and spontaneous mutants of Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2009 May;53(5):2100-9. doi: 10.1128/AAC.01197-08. Epub 2009 Feb 23. Antimicrob Agents Chemother. 2009. PMID: 19237648 Free PMC article.
-
Multi-omics comparisons of p-aminosalicylic acid (PAS) resistance in folC mutated and un-mutated Mycobacterium tuberculosis strains.Emerg Microbes Infect. 2019;8(1):248-261. doi: 10.1080/22221751.2019.1568179. Emerg Microbes Infect. 2019. PMID: 30866779 Free PMC article.
-
Phylogenetic and Structural Significance of Dihydrofolate Synthase (folC) Mutations in Drug-Resistant Mycobacterium tuberculosis.Microb Drug Resist. 2016 Oct;22(7):545-551. doi: 10.1089/mdr.2015.0193. Epub 2016 Apr 15. Microb Drug Resist. 2016. PMID: 27082669
-
Mycobacterium tuberculosis folate metabolism and the mechanistic basis for para-aminosalicylic acid susceptibility and resistance.Antimicrob Agents Chemother. 2015 Sep;59(9):5097-106. doi: 10.1128/AAC.00647-15. Epub 2015 Jun 1. Antimicrob Agents Chemother. 2015. PMID: 26033719 Free PMC article. Review.
-
The pharmacokinetics of para-aminosalicylic acid and its relationship to efficacy and intolerance.Br J Clin Pharmacol. 2020 Nov;86(11):2123-2132. doi: 10.1111/bcp.14395. Epub 2020 Jun 21. Br J Clin Pharmacol. 2020. PMID: 32470182 Free PMC article. Review.
Cited by
-
Breaking barriers: The potential of nanosystems in antituberculosis therapy.Bioact Mater. 2024 May 17;39:106-134. doi: 10.1016/j.bioactmat.2024.05.013. eCollection 2024 Sep. Bioact Mater. 2024. PMID: 38783925 Free PMC article. Review.
-
Discordance Between Phenotypic and WGS-Based Drug Susceptibility Testing Results for Some Anti-Tuberculosis Drugs: A Snapshot Study of Paired Mycobacterium tuberculosis Isolates with Small Genetic Distance.Infect Drug Resist. 2024 Aug 1;17:3289-3307. doi: 10.2147/IDR.S468997. eCollection 2024. Infect Drug Resist. 2024. PMID: 39108991 Free PMC article.
References
-
- World Health Organization . Global Tuberculosis Report 2021. Geneva: World Health Organization; 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

