Safe and effective off-the-shelf immunotherapy based on CAR.CD123-NK cells for the treatment of acute myeloid leukaemia
- PMID: 36335396
- PMCID: PMC9636687
- DOI: 10.1186/s13045-022-01376-3
Safe and effective off-the-shelf immunotherapy based on CAR.CD123-NK cells for the treatment of acute myeloid leukaemia
Abstract
Background: Paediatric acute myeloid leukaemia (AML) is characterized by poor outcomes in patients with relapsed/refractory disease, despite the improvements in intensive standard therapy. The leukaemic cells of paediatric AML patients show high expression of the CD123 antigen, and this finding provides the biological basis to target CD123 with the chimeric antigen receptor (CAR). However, CAR.CD123 therapy in AML is hampered by on-target off-tumour toxicity and a long "vein-to-vein" time.
Methods: We developed an off-the-shelf product based on allogeneic natural killer (NK) cells derived from the peripheral blood of healthy donors and engineered them to express a second-generation CAR targeting CD123 (CAR.CD123).
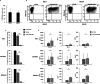
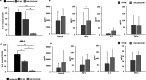
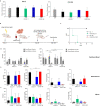
Results: CAR.CD123-NK cells showed significant anti-leukaemia activity not only in vitro against CD123+ AML cell lines and CD123+ primary blasts but also in two animal models of human AML-bearing immune-deficient mice. Data on anti-leukaemia activity were also corroborated by the quantification of inflammatory cytokines, namely granzyme B (Granz B), interferon gamma (IFN-γ) and tumour necrosis factor alpha (TNF-α), both in vitro and in the plasma of mice treated with CAR.CD123-NK cells. To evaluate and compare the on-target off-tumour effects of CAR.CD123-T and NK cells, we engrafted human haematopoietic cells (hHCs) in an immune-deficient mouse model. All mice infused with CAR.CD123-T cells died by Day 5, developing toxicity against primary human bone marrow (BM) cells with a decreased number of total hCD45+ cells and, in particular, of hCD34+CD38- stem cells. In contrast, treatment with CAR.CD123-NK cells was not associated with toxicity, and all mice were alive at the end of the experiments. Finally, in a mouse model engrafted with human endothelial tissues, we demonstrated that CAR.CD123-NK cells were characterized by negligible endothelial toxicity when compared to CAR.CD123-T cells.
Conclusions: Our data indicate the feasibility of an innovative off-the-shelf therapeutic strategy based on CAR.CD123-NK cells, characterized by remarkable efficacy and an improved safety profile compared to CAR.CD123-T cells. These findings open a novel intriguing scenario not only for the treatment of refractory/resistant AML patients but also to further investigate the use of CAR-NK cells in other cancers characterized by highly difficult targeting with the most conventional T effector cells.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicting financial interests to disclose.
Figures





References
-
- Fiorenza S, Turtle CJ. CAR-T cell therapy for acute myeloid leukemia: preclinical rationale, current clinical progress, and barriers to success. BioDrugs. 2021;35:281–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

