Targeting the brain 5-HT7 receptor to prevent hypomyelination in a rodent model of perinatal white matter injuries
- PMID: 36335540
- PMCID: PMC10033587
- DOI: 10.1007/s00702-022-02556-8
Targeting the brain 5-HT7 receptor to prevent hypomyelination in a rodent model of perinatal white matter injuries
Abstract
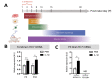
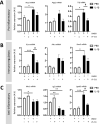
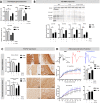
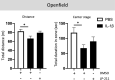
Approximately 15 million babies are born prematurely every year and many will face lifetime motor and/or cognitive deficits. Children born prematurely are at higher risk of developing perinatal brain lesions, especially white matter injuries (WMI). Evidence in humans and rodents demonstrates that systemic inflammation-induced neuroinflammation, including microglial and astrocyte reactivity, is the prominent processes of WMI associated with preterm birth. Thus, a new challenge in the field of perinatal brain injuries is to develop new neuroprotective strategies to target neuroinflammation to prevent WMI. Serotonin (5-HT) and its receptors play an important role in inflammation, and emerging evidence indicates that 5-HT may regulate brain inflammation by the modulation of microglial reactivity and astrocyte functions. The present study is based on a mouse model of WMI induced by intraperitoneal (i.p.) injections of IL-1β during the first 5 days of life. In this model, certain key lesions of preterm brain injuries can be summarized by (i) systemic inflammation, (ii) pro-inflammatory microglial and astrocyte activation, and (iii) inhibition of oligodendrocyte maturation, leading to hypomyelination. We demonstrate that Htr7 mRNA (coding for the HTR7/5-HT7 receptor) is significantly overexpressed in the anterior cortex of IL-1β-exposed animals, suggesting it as a potential therapeutic target. LP-211 is a specific high-affinity HTR7 agonist that crosses the blood-brain barrier (BBB). When co-injected with IL-1β, LP-211 treatment prevented glial reactivity, the down-regulation of myelin-associated proteins, and the apparition of anxiety-like phenotypes. Thus, HTR7 may represent an innovative therapeutic target to protect the developing brain from preterm brain injuries.
Keywords: Astrocyte; HTR7; Microglia; Myelination; Neurodevelopmental disorders; Preterm birth; Serotonin.
© 2022. The Author(s).
Conflict of interest statement
Not applicable.
Figures



References
-
- Basu S, et al. Elevated plasma and cerebrospinal fluid interleukin-1 beta and tumor necrosis factor-alpha concentration and combined outcome of death or abnormal neuroimaging in preterm neonates with early-onset clinical sepsis. J Perinatol. 2015;35(10):855–861. doi: 10.1038/jp.2015.86. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

