KBTBD13 is a novel cardiomyopathy gene
- PMID: 36335629
- PMCID: PMC10100581
- DOI: 10.1002/humu.24499
KBTBD13 is a novel cardiomyopathy gene
Abstract
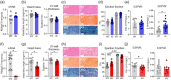
KBTBD13 variants cause nemaline myopathy type 6 (NEM6). The majority of NEM6 patients harbors the Dutch founder variant, c.1222C>T, p.Arg408Cys (KBTBD13 p.R408C). Although KBTBD13 is expressed in cardiac muscle, cardiac involvement in NEM6 is unknown. Here, we constructed pedigrees of three families with the KBTBD13 p.R408C variant. In 65 evaluated patients, 12% presented with left ventricle dilatation, 29% with left ventricular ejection fraction< 50%, 8% with atrial fibrillation, 9% with ventricular tachycardia, and 20% with repolarization abnormalities. Five patients received an implantable cardioverter defibrillator, three cases of sudden cardiac death were reported. Linkage analysis confirmed cosegregation of the KBTBD13 p.R408C variant with the cardiac phenotype. Mouse studies revealed that (1) mice harboring the Kbtbd13 p.R408C variant display mild diastolic dysfunction; (2) Kbtbd13-deficient mice have systolic dysfunction. Hence, (1) KBTBD13 is associated with cardiac dysfunction and cardiomyopathy; (2) KBTBD13 should be added to the cardiomyopathy gene panel; (3) NEM6 patients should be referred to the cardiologist.
Keywords: KBTBD13; NEM6; cardiomyopathy; congenital myopathy.
© 2022 The Authors. Human Mutation published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Garibaldi, M. , Fattori, F. , Bortolotti, C. A. , Brochier, G. , Labasse, C. , Verardo, M. , Servian‐Morilla, E. , Gibellini, L. , Pinti, M. , Di Rocco, G. , Raffa, S. , Pennisi, E. M. , Bertini, E. S. , Paradas, C. , Romero, N. B. , & Antonini, G. (2018). Core‐rod myopathy due to a novel mutation in BTB/POZ domain of KBTBD13 manifesting as late onset LGMD. Acta Neuropathologica Communications, 6(1):94. 10.1186/s40478-018-0595-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

