Nrf2 deficiency deteriorates diabetic kidney disease in Akita model mice
- PMID: 36335764
- PMCID: PMC9641024
- DOI: 10.1016/j.redox.2022.102525
Nrf2 deficiency deteriorates diabetic kidney disease in Akita model mice
Abstract
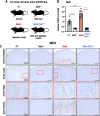
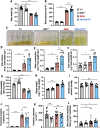
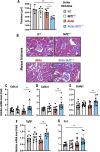
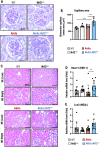
Oxidative stress is an essential component in the progression of diabetic kidney disease (DKD), and the transcription factor NF-E2-related factor-2 (Nrf2) plays critical roles in protecting the body against oxidative stress. To clarify the roles of Nrf2 in protecting against DKD, in this study we prepared compound mutant mice with diabetes and loss of antioxidative defense. Specifically, we prepared compound Ins2Akita/+ (Akita) and Nrf2 knockout (Akita::Nrf2-/-) or Akita and Nrf2 induction (Akita::Keap1FA/FA) mutant mice. Eighteen-week-old Akita::Nrf2-/- mice showed more severe diabetic symptoms than Akita mice. In the Akita::Nrf2-/- mouse kidneys, the glomeruli showed distended capillary loops, suggesting enhanced mesangiolysis. Distal tubules showed dilation and an increase in 8-hydroxydeoxyguanosine-positive staining. In the Akita::Nrf2-/- mouse kidneys, the expression of glutathione (GSH) synthesis-related genes was decreased, and the actual GSH level was decreased in matrix-assisted laser desorption/ionization mass spectrometry imaging analysis. Akita::Nrf2-/- mice exhibited severe inflammation and enhancement of infiltrated macrophages in the kidney. To further examine the progression of DKD, we compared forty-week-old Akita mouse kidney compounds with Nrf2-knockout or Nrf2 mildly induced (Akita::Keap1FA/FA) mice. Nrf2-knockout Akita (Akita::Nrf2-/-) mice displayed severe medullary cast formation, but the formation was ameliorated in Akita::Keap1FA/FA mice. Moreover, in Akita::Keap1FA/FA mice, tubule injury and inflammation-related gene expression were significantly suppressed, which was evident in Akita::Nrf2-/- mouse kidneys. These results demonstrate that Nrf2 contributes to the protection of the kidneys against DKD by suppressing oxidative stress and inflammation.
Keywords: Diabetic kidney disease; Glutathione; Inflammation; MALDI-MSI; Nrf2; Oxidative stress.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










References
-
- Nitta K., Goto S., Masakane I., Hanafusa N., Taniguchi M., Hasegawa T., Nakai S., Wada A., Hamano T., Hoshino J., Joki N., Abe M., Yamamoto K., Nakamoto H., Maeno K., Kawata T., Oyama C., Seino K., Sato T., Sato S., Ito M., Kazama J., Ueda A., Saito O., Ando T., Ogawa T., Kumagai H., Terawaki H., Ando R., Abe M., Kashiwagi T., Hamada C., Shibagaki Y., Hirawa N., Shimada H., Ishida Y., Yokoyama H., Miyazaki R., Fukasawa M., Kamijyo Y., Matsuoka T., Kato A., Mori N., Ito Y., Kasuga H., Koyabu S., Arimura T., Hashimoto T., Inaba M., Hayashi T., Yamakawa T., Nishi S., Fujimori A., Yoneda T., Negi S., Nakaoka A., Ito T., Sugiyama H., Masaki T., Nitta Y., Okada K., Yamanaka M., Kan M., Ota K., Tamura M., Mitsuiki K., Ikeda Y., Nishikido M., Miyata A., Tomo T., Fujimoto S., Nosaki T., Oshiro Y. C. on behalf of the Japanese Society for Dialysis Therapy Renal Data Registry, Annual dialysis data report for 2018, JSDT Renal Data Registry: survey methods, facility data, incidence, prevalence, and mortality. Renal Replacement Therapy. 2020;6(1):41.
-
- Saran R., Robinson B., Abbott K.C., Bragg-Gresham J., Chen X., Gipson D., Gu H., Hirth R.A., Hutton D., Jin Y., Kapke A., Kurtz V., Li Y., McCullough K., Modi Z., Morgenstern H., Mukhopadhyay P., Pearson J., Pisoni R., Repeck K., Schaubel D.E., Shamraj R., Steffick D., Turf M., Woodside K.J., Xiang J., Yin M., Zhang X., Shahinian V. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2020;75(1 Suppl 1):A6–A7. - PubMed
-
- Kdoqi KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am. J. Kidney Dis. 2007;49(2 Suppl 2):S12–S154. - PubMed
-
- Gurley S.B., Coffman T.M. The renin-angiotensin system and diabetic nephropathy. Semin. Nephrol. 2007;27(2):144–152. - PubMed
-
- Balakumar P., Arora M.K., Ganti S.S., Reddy J., Singh M. Recent advances in pharmacotherapy for diabetic nephropathy: current perspectives and future directions. Pharmacol. Res. 2009;60(1):24–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

