Overview of fuzuloparib in the treatment of ovarian cancer: background and future perspective
- PMID: 36335989
- PMCID: PMC9634097
- DOI: 10.3802/jgo.2022.33.e86
Overview of fuzuloparib in the treatment of ovarian cancer: background and future perspective
Abstract
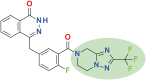
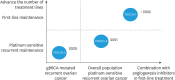
Over the last decade, clinical trials using various poly ADP ribose polymerase (PARP) inhibitors on patients with ovarian cancer have shown promising results. The introduction of PARP inhibitors has changed the treatment landscape and improved outcomes for patients with ovarian cancer. Fuzuloparib, developed by Jiangsu Hengrui Pharmaceuticals Co., Ltd., is a novel orally available small molecule PARP inhibitor. By introducing the trifluoromethyl group into chemical structure, fuzuloparib exhibits higher stability and lower inter-individual variability than other PARP inhibitors. Several clinical trials (FZOCUS series and others) have been carried out to assess the efficacy and safety of fuzuloparib through different lines of treatments for advanced or recurrent ovarian cancer in both treatment and maintenance. Here, we present the most recent data from these studies, discuss current progress and potential future directions.
Keywords: Fuzuloparib; Maintenance Therapy; Ovarian Cancer; PARP Inhibitor.
© 2022. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- International Agency for Research on Cancer (IARC), World Health Organization. Cancer today [Internet] Geneva: World Health Organization; 2020. [cited 2022 Oct 10]. Available from: https://gco.iarc.fr/today/home.
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–1253. - PubMed