Inhibition of Heat Shock Factor 1 Signaling Decreases Hepatoblastoma Growth via Induction of Apoptosis
- PMID: 36336065
- PMCID: PMC9887635
- DOI: 10.1016/j.ajpath.2022.10.006
Inhibition of Heat Shock Factor 1 Signaling Decreases Hepatoblastoma Growth via Induction of Apoptosis
Abstract
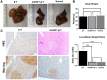
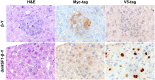
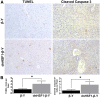
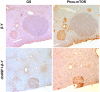
Although rare compared with adult liver cancers, hepatoblastoma (HB) is the most common pediatric liver malignancy, and its incidence is increasing. Currently, the treatment includes surgical resection with or without chemotherapy, and in severe cases, liver transplantation in children. The effort to develop more targeted, HB-specific therapies has been stymied by the lack of fundamental knowledge about HB biology. Heat shock factor 1 (HSF1), a transcription factor, is a canonical inducer of heat shock proteins, which act as chaperone proteins to prevent or undo protein misfolding. Recent work has shown a role for HSF1 in cancer beyond the canonical heat shock response. The current study found increased HSF1 signaling in HB versus normal liver. It showed that less differentiated, more embryonic tumors had higher levels of HSF1 than more differentiated, more fetal-appearing tumors. Most strikingly, HSF1 expression levels correlated with mortality. This study used a mouse model of HB to test the effect of inhibiting HSF1 early in tumor development on cancer growth. HSF1 inhibition resulted in fewer and smaller tumors, suggesting HSF1 is needed for aggressive tumor growth. Moreover, HSF1 inhibition also increased apoptosis in tumor foci. These data suggest that HSF1 may be a viable pharmacologic target for HB treatment.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Pham T.A., Gallo A.M., Concepcion W., Esquivel C.O., Bonham C.A. Effect of liver transplant on long-term disease-free survival in children with hepatoblastoma and hepatocellular cancer. JAMA Surg. 2015;150:1150–1158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

