Cardiac troponin release following coronary artery bypass grafting: mechanisms and clinical implications
- PMID: 36337034
- PMCID: PMC9897191
- DOI: 10.1093/eurheartj/ehac604
Cardiac troponin release following coronary artery bypass grafting: mechanisms and clinical implications
Abstract
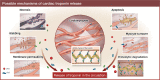
The use of biomarkers is undisputed in the diagnosis of primary myocardial infarction (MI), but their value for identifying MI is less well studied in the postoperative phase following coronary artery bypass grafting (CABG). To identify patients with periprocedural MI (PMI), several conflicting definitions of PMI have been proposed, relying either on cardiac troponin (cTn) or the MB isoenzyme of creatine kinase, with or without supporting evidence of ischaemia. However, CABG inherently induces the release of cardiac biomarkers, as reflected by significant cTn concentrations in patients with uncomplicated postoperative courses. Still, the underlying (patho)physiological release mechanisms of cTn are incompletely understood, complicating adequate interpretation of postoperative increases in cTn concentrations. Therefore, the aim of the current review is to present these potential underlying mechanisms of cTn release in general, and following CABG in particular (Graphical Abstract). Based on these mechanisms, dissimilarities in the release of cTnI and cTnT are discussed, with potentially important implications for clinical practice. Consequently, currently proposed cTn biomarker cut-offs by the prevailing definitions of PMI might warrant re-assessment, with differentiation in cut-offs for the separate available assays and surgical strategies. To resolve these issues, future prospective studies are warranted to determine the prognostic influence of biomarker release in general and PMI in particular.
Keywords: Cardiac surgery; Cardiac troponin; Coronary artery bypass grafting; Myocardial infarction; Periprocedural myocardial infarction.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.M.A.M. has received nonfinancial support from Abbott Diagnostics and Roche Diagnostics. These manufacturers had no role in the preparation of this review, or the decision to submit the article for publication. A.W.J.V.H. reports his institution received unrestricted grants from Abbott, Roche Medtronic, Boehringer Ingelheim and Astra Zeneca, unrelated to this work. All other authors have no conflicts of interest to declare.
Figures


References
-
- Heuts S, Denessen EJS, Daemen JHT, Vroemen WHM, Sels JW, Segers P, et al. Meta-Analysis evaluating high-sensitivity cardiac troponin T kinetics after coronary artery bypass grafting in relation to the current definitions of myocardial infarction. Am J Cardiol 2022;163:25–31. - PubMed
-
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–269. - PubMed
-
- Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol 2013;62:1563–1570. - PMC - PubMed
-
- Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Eur Heart J 2018;39:2192–2207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

