6,7-Di-hydro-5 H-pyrrolo-[1,2- a]imidazole
- PMID: 36337149
- PMCID: PMC9462221
- DOI: 10.1107/S2414314620006811
6,7-Di-hydro-5 H-pyrrolo-[1,2- a]imidazole
Abstract
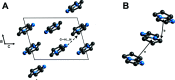
The crystal structure of 6,7-di-hydro-5H-pyrrolo-[1,2-a]imidazole, C6H8N2, at 100 K has monoclinic (P21/n) symmetry. The mol-ecule adopts an envelope conformation of the pyrrolidine ring, which might help for the relief torsion tension. The crystal cohesion is achieved by C-H⋯N hydrogen bonds. Inter-estingly, this fused ring system provides protection of the α-C atom (attached to the non-bridging N atom of the imidazole ring), which provides stability that is of inter-est with respect to electrochemical properties as electrolytes for fuel cells and batteries, and electrodeposition.
Keywords: crystal structure; imidazole derivative; ring puckering analysis.
© Morales-Collazo et al. 2020.
Figures


References
-
- Dziubek, K. F. & Katrusiak, A. (2011). Phys. Chem. Chem. Phys. 13, 15428–15431. - PubMed
-
- Higashi, T. (2001). ABSCOR. Rigaku Corporation, Tokyo, Japan.
-
- Huang, K., Song, T., Morales-Collazo, O., Jia, H. & Brennecke, J. F. (2017). J. ECS. 164, F1448–F1459.
-
- Kan, H.-C., Tseng, M.-C. & Chu, Y. H. (2007). Tetrahedron, 63, 1644–1653.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
