The Genetics of Ethambutol-Induced Optic Neuropathy: A Narrative Review
- PMID: 36337233
- PMCID: PMC9635551
- DOI: 10.1080/01658107.2022.2100916
The Genetics of Ethambutol-Induced Optic Neuropathy: A Narrative Review
Abstract
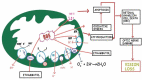
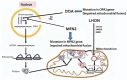
Tuberculosis (TB) is a global health problem with the major brunt of disease occurring in developing countries. The cornerstone of treatment of TB is anti-tubercular therapy (ATT), which includes rifampicin, isoniazid, pyrazinamide and ethambutol. Because of emerging drug resistance, treatment failures, defaulters and increasing incidence of disseminated and extrapulmonary TB, the guidelines have been modified in some countries. Ethambutol is prescribed for longer times (in some cases >8 months) and hence the incidence of ethambutol-induced optic neuropathy (EtON) is expected to rise. The fundamental question which needs explanation is why only a small subset of patients on ethambutol are prone to develop loss of vision. This review focuses on available genetic studies which provide evidence that mitochondria are the likely substrates involved in the final pathway of reactive oxidative damage of the papillo-macular bundle. Genetic analysis of mitochondrial mutations encoding genes involved in oxidative phosphorylation pathways may help in isolating the subset of patients who are genetically susceptible. If the groups having high risk of developing EtON are recognised then prolonged duration of ethambutol treatment can be avoided in these susceptible individuals. A better understanding of the pathophysiology will also pave the way for the development of management strategies in this condition.
Keywords: Tuberculosis; ethambutol; mitochondria; optic neuropathy; pharmacogenomics.
© 2022 Taylor & Francis Group, LLC.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
-
- World Health Organization, Stop TB Initiative (World Health Organization) . Treatment of Tuberculosis: Guidelines. WHO Press: World Health Organization; 2010.
-
- Chaudhuri. Recent changes in technical and operational guidelines for tuberculosis control programme in India - 2016: a paradigm shift in tuberculosis control. J Assoc Chest Physicians. 2017;5(1):1.
Publication types
LinkOut - more resources
Full Text Sources
