Red propolis exhibits chemopreventive effect associated with antiproliferative and anti-inflammatory activities
- PMID: 36337250
- PMCID: PMC9618114
- DOI: 10.1093/toxres/tfac049
Red propolis exhibits chemopreventive effect associated with antiproliferative and anti-inflammatory activities
Abstract
Introduction: Red propolis is synthetized from exudates of Dalbergia ecastophyllum (L) Taub. and Symphonia globulifera L.f., presents isoflavones, guttiferone E, xanthochymol, and oblongifolin B and has anti-inflammatory, antioxidant, and antiproliferative activities.
Objectives: This study aimed to evaluate the antigenotoxic and anticarcinogenic potential of red propolis hydroalcoholic extract (RPHE) in rodents.
Methods: The influence of RPHE in doxorubicin (DXR)-induced genotoxicity was investigated through the micronucleus test in Swiss mice. Blood samples were also collected to investigate oxidative stress, hepatotoxicity, and nephrotoxicity. Was investigated the influence of RPHE in 1,2-dimethylhydrazine (DMH)-induced aberrant crypt foci, as well as its influence in proliferating cell nuclear antigen (PCNA) and the cyclooxygenase-2 (COX-2) expression in colon of rats, by immunohistochemistry.
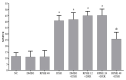
Results: The results showed that RPHE (48 mg/kg) reduced DXR-induced genotoxicity. Animals treated with DXR showed significantly lower GSH serum levels in comparison to the negative control. RPHE treatments did not attenuated significantly the DXR-induced GSH depletion. No difference was observed in cytotoxicity parameters of mice hematopoietic tissues between the treatment groups, as well as the biochemical parameters of hepatotoxicity and nephrotoxicity. RPHE (12 mg/kg) reduced the DMH-induced carcinogenicity and toxicity, as well as DMH-induced PCNA and COX-2 expression in colon tissue.
Conclusion: Therefore, was observed that the RPHE has chemopreventive effect, associated to antiproliferative and anti-inflammatory activities.
Keywords: anti-inflammatory; anticarcinogenic; antiproliferative; chemoprevention; propolis.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Wild CP, Weiderpass E, Stewart BW. World cancer report: cancer research for cancer prevention. France: Lyon; 2020
-
- Jin K, Ren C, Liu Y, Lan H, Wang Z. An update on colorectal cancer microenvironment, epigenetic and immunotherapy. Int Immunopharmacol. 2020:89(Pt A):1–8. - PubMed
-
- Zhou E, Rifkin S. Colorectal cancer and diet: risk versus prevention, is diet an intervention? Gastroenterol Clin N Am. 2021:50(1):101–111. - PubMed
-
- Huang XM, Yang ZJ, Xie Q, Zhang ZK, Zhang H, Ma JY. Natural products for treating colorectal cancer: a mechanistic review. Biomed Pharmacother. 2019:117:109142. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

