(E)-1-(2-Hy-droxy-4,6-di-meth-oxy-phen-yl)-3-(naph-thalen-1-yl)prop-2-en-1-one
- PMID: 36337459
- PMCID: PMC9635433
- DOI: 10.1107/S2414314622009324
(E)-1-(2-Hy-droxy-4,6-di-meth-oxy-phen-yl)-3-(naph-thalen-1-yl)prop-2-en-1-one
Abstract
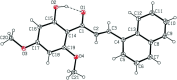
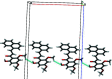
In the title compound, C21H18O4, the relative conformation of the C=C and C=O double bonds in the central enone group is s-cisoid; there is a trans configuration about the C=C bond. The dihedral angle formed by the naphthalene ring system and the benzene ring is 16.80 (2)°. The meth-oxy groups at the ortho and para positions of the benzene ring are tilted to the ring by 169.8 (1) and 174.5 (1)°, respectively. The hy-droxy group in the benzene ring participates in an intra-molecular O-H⋯O hydrogen bond. In the crystal, C-H⋯O inter-actions link mol-ecules into linear chains along the a-axis direction.
Keywords: C—H⋯O interactions; O—H⋯O hydrogen bond; chalcone; crystal structure.
© Dongsoo Koh 2022.
Figures
References
-
- Bruker (2012). APEX2, SAINT and SADABS. Bruker AXS Inc. Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Research Materials
