5-{[4-(Di-methyl-amino)-phen-yl]ethyn-yl}pyrimidine-1,2,3,5-tetra-fluoro-4,6-di-iodo-benzene (1/2)
- PMID: 36337692
- PMCID: PMC9462022
- DOI: 10.1107/S2414314622003807
5-{[4-(Di-methyl-amino)-phen-yl]ethyn-yl}pyrimidine-1,2,3,5-tetra-fluoro-4,6-di-iodo-benzene (1/2)
Abstract
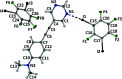
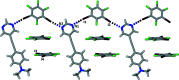
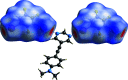
The treatment of 5-{[4-(di-methyl-amino)-phen-yl]ethyn-yl}pyrimidine with a threefold excess of 1,2,3,5-tetra-fluoro-4,6-di-iodo-benzene in di-chloro-methane solution led to the formation of the unexpected 1:2 title co-crystal, C14H13N3·2CF4I2. In the extended structure, two unique C-I⋯N halogen bonds from one of the 1,2,3,5-tetra-fluoro-4,6-di-iodo-benzene mol-ecules to the pyrimidine N atoms of the 5-{[4-(di-methyl-amino)-phen-yl]ethyn-yl}pyrimidine mol-ecule generate [110] chains and layers of these chains are π-stacked along the a-axis direction. The second 1,2,3,5-tetra-fluoro-4,6-di-iodo-benzene mol-ecule resides in channels formed parallel to the a-axis direction between stacks of 5-{[4-(di-methyl-amino)-phen-yl]ethyn-yl}pyrimidine mol-ecules and inter-acts with them via C-I⋯π(alkyne) contacts.
Keywords: 1,2,3,5-tetrafluoro-4,6-diiodobenzene solvate; co-crystal; crystal structure; halogen bonding.
© Bosch and Bowling 2022.
Figures


References
-
- Barbour, L. J. (2020). J. Appl. Cryst. 53, 1141–1146.
-
- Bruker (2014). SMART, SAINT, and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Mir, N. A., Dubey, R. & Desiraju, G. R. (2019). Acc. Chem. Res. 52, 2210–2220. - PubMed
-
- Nwachukwu, C. I., Patton, L. J., Bowling, N. P. & Bosch, E. (2020). Acta Cryst. C76, 458–467. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
