Changes in the Characteristics and Initial Treatments of Pulmonary Hypertension Between 2008 and 2020 in Japan
- PMID: 36338395
- PMCID: PMC9627817
- DOI: 10.1016/j.jacasi.2022.02.011
Changes in the Characteristics and Initial Treatments of Pulmonary Hypertension Between 2008 and 2020 in Japan
Abstract
Background: Pulmonary arterial hypertension (PAH) is a rare, progressive disease. The treatment landscape for PAH in Japan has evolved considerably in recent years, but there is limited knowledge of the changes in treatment practices or patient characteristics.
Objectives: The aim of this study was to evaluate the changes in characteristics and initial treatments for PAH in Japan over time.
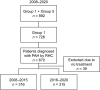
Methods: This study used data from the Japan Pulmonary Hypertension Registry (JAPHR) to compare patient characteristics and treatment practices between 2008-2015 (n = 316) and 2016-2020 (n = 315).
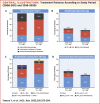
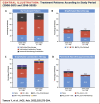
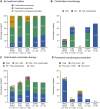
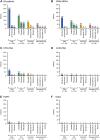
Results: The mean ± standard deviation age at diagnosis increased from 47.9 ± 16.7 years in 2008-2015 to 52.7 ± 16.9 years in 2016-2020. The mean pulmonary arterial pressure decreased from 45.4 ± 15.0 to 38.6 ± 13.1 mm Hg. Idiopathic/hereditary PAH was the most common etiology in both periods (50.0% and 51.1%, respectively). The proportion of patients prescribed oral/inhaled combination therapies increased from 47.8% to 57.5%. Oral/inhaled combination therapies were frequently prescribed to patients with congenital heart disease-related PAH (81.8%). There was no significant trend in prescribing practices based on French low-risk criteria: among patients with 0, 1, 2, 3, or 4 criteria, 53.8%, 68.8%, 52.8%, 66.7%, and 39.4% were prescribed oral/inhaled combination therapies, and 0%, 16.7%, 27.0%, 17.3%, and 15.2% were prescribed oral/inhaled monotherapies. Macitentan, tadalafil, selexipag, and epoprostenol were the most frequently prescribed drugs.
Conclusions: The severity of PAH decreased over time in Japan. Oral/inhaled combination therapies were generally preferred. Physicians generally prescribed therapies after considering the patients' hemodynamics and clinical severity. (Japan Pulmonary Hypertension Registry [JAPHR]; UMIN000026680).
Keywords: 6MWD, 6-minute walk distance; AMED, Agency for Medical Research and Development; BNP, brain natriuretic peptide; CHD-PAH, congenital heart disease–related pulmonary arterial hypertension; CTD-PAH, connective tissue disease–related pulmonary arterial hypertension; ERA, endothelin receptor antagonist; HPAH, hereditary pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; JAPHR, Japan Pulmonary Hypertension Registry; Japan; NO, nitric oxide; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PGI2, prostacyclin; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; PoPH, portopulmonary hypertension; mPAP, mean pulmonary arterial pressure; mRAP, mean right arterial pressure; pulmonary arterial hypertension; risk criteria; treatment.
© 2022 The Authors.
Conflict of interest statement
This study was funded by a Health Labor Sciences Research Grant, Japan and AMED under grant number JP18lk1601003h0001. The funding body contributed to study design and data collection. Editorial support was funded by Janssen Pharmaceutical. Dr Tamura has received remuneration from Janssen and Daiichi Sankyo; research funds from Mochida; and is affiliated with the Pulmonary Hypertension Center, which is supported by an endowment from Nippon Shinyaku. Dr Kumamaru, Dr Miyata, and Ms Nishimura are affiliated with the Department of Health Quality Assessment at the University of Tokyo, a social collaboration department supported by the National Clinical Database, Johnson & Johnson, and Nipro. Dr Miyata is also affiliated with the Department of Health Policy and Management School of Medicine at Keio University that is conducting joint research with the National Clinical Database. Dr Matsubara has received remuneration from Janssen, Bayer, Pfizer Japan, Nippon Shinyaku, Kaneka Medix, GlaxoSmithKline, United Therapeutics, and Mochida; and research funds from Nippon Shinyaku. Dr Hirata has received remuneration from Takeda and Kowa; commissioned, joint, or physician-led research with Daiichi Sankyo, Janssen, Sysmex, and Terumo; scholarship donations from Merck Sharp & Dohme, Janssen, Abbott, Otsuka, Kowa, Sanofi, Takeda, Toa Eiyo, Nippon Shinyaku, Nippon Boehringer Ingelheim, Nihon Medi-Physics, Novartis, Bayer, Biotronik, and Fujifilm Toyama Chemical; and is affiliated with the Division of Cardiovascular Medicine Department of Internal Medicine, which is supported by endowments from Abbott, Medtronic, and Sysmex. Dr Tsujino has received remuneration from Nippon Shinyaku and Janssen; and is affiliated with the First Department of Medicine, which is supported by endowments from Nippon Shinyaku, Nippon Boehringer Ingelheim, and Mochida. Dr Suda is affiliated with the Department of Respirology, which is supported by endowments from Nippon Shinyaku and Janssen. Dr Tatsumi has received remuneration from Janssen. Mr Sigel and Mr Takano are employees of Janssen Pharmaceutical. Dr Inami has reported that he has no relationships relevant to the contents of this paper to disclose.
Figures
References
-
- Benza R.L., Miller D.P., Barst R.J., Badesch D.B., Frost A.E., McGoon M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. - PubMed
-
- Kozu K., Sugimura K., Ito M., et al. Current status of long-term prognosis among all subtypes of pulmonary hypertension in Japan. Int J Cardiol. 2020;300:228–235. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

