Tumor microenvironment and exosomes in brain metastasis: Molecular mechanisms and clinical application
- PMID: 36338717
- PMCID: PMC9631487
- DOI: 10.3389/fonc.2022.983878
Tumor microenvironment and exosomes in brain metastasis: Molecular mechanisms and clinical application
Abstract
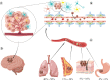
Metastasis is one of the important biological features of malignant tumors and one of the main factors responsible for poor prognosis. Although the widespread application of newer clinical technologies and their continuous development have significantly improved survival in patients with brain metastases, there is no uniform standard of care. More effective therapeutic measures are therefore needed to improve prognosis. Understanding the mechanisms of tumor cell colonization, growth, and invasion in the central nervous system is of particular importance for the prevention and treatment of brain metastases. This process can be plausibly explained by the "seed and soil" hypothesis, which essentially states that tumor cells can interact with various components of the central nervous system microenvironment to produce adaptive changes; it is this interaction that determines the development of brain metastases. As a novel form of intercellular communication, exosomes play a key role in the brain metastasis microenvironment and carry various bioactive molecules that regulate receptor cell activity. In this paper, we review the roles and prospects of brain metastatic tumor cells, the brain metastatic tumor microenvironment, and exosomes in the development and clinical management of brain metastases.
Keywords: brain tumor; exosomes; metastases; tumor cell; tumor microenvironment.
Copyright © 2022 Aili, Maimaitiming, Qin, Ji, Fan, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Lee HH, Chen CH, Chuang HY, Huang YW, Huang MY. Brain surgery in combination with tyrosine kinase inhibitor and whole brain radiotherapy for epidermal growth factor receptor-mutant non-small-cell lung cancer with brain metastases. Sci Rep (2019) 9(1):16834. doi: 10.1038/s41598-019-53456-z - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

