Stimuli-responsive transdermal microneedle patches
- PMID: 36338772
- PMCID: PMC9635273
- DOI: 10.1016/j.mattod.2021.03.012
Stimuli-responsive transdermal microneedle patches
Abstract
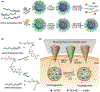
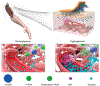
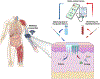
Microneedle (MN) patches consisting of miniature needles have emerged as a promising tool to perforate the stratum corneum and translocate biomolecules into the dermis in a minimally invasive manner. Stimuli-responsive MN patches represent emerging drug delivery systems that release cargos on-demand as a response to internal or external triggers. In this review, a variety of stimuli-responsive MN patches for controlled drug release are introduced, covering the mechanisms of action toward different indications. Future opportunities and challenges with respect to clinical translation are also discussed.
Conflict of interest statement
Competing interests Z.G. is a scientific co-founder of Zenomics Inc. and Zcapsule Inc. R.L. is a scientific advisor to Zenomic Inc. and Zcapsule Inc. For a list of entities with which R.L. is involved, compensated or uncompensated, see https://tinyurl.com/RLCOINBME. The remaining authors declare no competing interests.
Figures




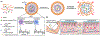





References
Grants and funding
LinkOut - more resources
Full Text Sources
