Tetra-kis(di-cyclo-hexyl-amido)-zirconium(IV)
- PMID: 36338916
- PMCID: PMC9462282
- DOI: 10.1107/S2414314620011451
Tetra-kis(di-cyclo-hexyl-amido)-zirconium(IV)
Abstract
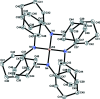
The reaction of ZrCl4 with three equivalents of LiNCy2 (Cy is cyclo-hex-yl) resulted in the formation of tris-(di-cyclo-hexyl-amido)-zirconium chloride and the title compound, [Zr(C12H22N)4]. The latter is isotypic with its cerium(IV) analogue and crystallizes with three independent mol-ecules in the asymmetric unit. One mol-ecule is located about a twofold rotation axis, and the other two on fourfold inversion axes. In each mol-ecule, the ZrIV atom has a distorted tetra-hedral coordination environment. The crystal under investigation was twinned by inversion in a 1:1 ratio.
Keywords: amide; crystal structure; isotypism; zirconium.
© Frerichs et al. 2020.
Figures
References
-
- Adler, C., Bekurdts, A., Haase, D., Saak, W., Schmidtmann, M. & Beckhaus, R. (2014b). Eur. J. Inorg. Chem. pp. 1289–1302.
-
- Adler, C., Tomaschun, G., Schmidtmann, M. & Beckhaus, R. (2014a). Organometallics, 33, 7011–7014.
-
- Brandenburg, K. & Putz, H. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
-
- Bruker (2015). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Diamond, G. M., Jordan, R. F. & Petersen, J. L. (1996). Organometallics, 15, 4030–4037.
LinkOut - more resources
Full Text Sources
