1,1'-Methyl-enebis(4,4'-bipyridin-1-ium) dibromide
- PMID: 36338934
- PMCID: PMC9462032
- DOI: 10.1107/S2414314622005260
1,1'-Methyl-enebis(4,4'-bipyridin-1-ium) dibromide
Abstract
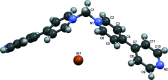
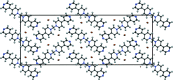
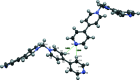
The asymmetric unit of the title salt, C21H18N4 2+·2Br-, comprises half of the mol-ecule and a bromide ion. The chevron-shaped cations stack as columns in the [001] direction with suitable inter-molecular distance for π-π inter-actions. These cationic columns are further stabilized by inter-columnar C-H⋯N hydrogen bonding with the bromide ions distributed between them.
Keywords: crystal structure; hydrogen bonding; pyridinium; π–π interactions.
© Schuster et al. 2022.
Figures




References
-
- Blanco, V., Chas, M., Abella, D., Peinador, C. & Quintela, J. M. (2007). J. Am. Chem. Soc. 129, 13978–13986. - PubMed
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Martinez, C. R. & Iverson, B. L. (2012). Chem. Sci. 3, 2191–2201.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
