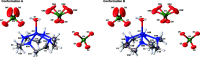Aqua-(1,4,7,10-tetra-aza-cyclo-dodeca-ne)zinc(II) bis-(perchlorate)
- PMID: 36339098
- PMCID: PMC9462334
- DOI: 10.1107/S2414314621003977
Aqua-(1,4,7,10-tetra-aza-cyclo-dodeca-ne)zinc(II) bis-(perchlorate)
Abstract
The cationic ZnII part of aqua-(1,4,7,10-tetra-aza-cyclo-dodeca-ne)zinc(II) bis-(perchlorate), [Zn(C8H20N4)(H2O)](ClO4)2, exhibits a slightly distorted square-pyramidal coordination environment with a water mol-ecule in the apical position. In the crystal, the macrocyclic ring alternates between two conformations with equal occupancies. Two of the three perchlorate anions are situated about a twofold rotation axis, and one of them shows disorder of the O atoms with occupancies of 0.62 (7) and 0.38 (7). In the crystal, the complexes are connected by inter-molecular hydrogen bonding via the perchlorate anions.
Keywords: crystal structure; cyclen; zinc(II) complex.
© Ichimaru et al. 2021.
Figures
References
-
- Addison, W. A., Rao, N. T., Reedijk, J., van Rijn, J. & Verschoor, C. G. (1984). J. Chem. Soc. Dalton Trans. pp. 1349–1356.
-
- Bazzicalupi, C., Bencini, A., Bianchi, A., Fusi, V., Paoletti, P. & Valtancoli, B. (1995). J. Chem. Soc. Chem. Commun. pp. 1555–1556.
-
- Chen, X.-M., Deng, Q.-Y., Wang, G. & Xu, Y.-J. (1994). Polyhedron, 13, 3085–3089.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Kato, M. & Ito, T. (1985). Inorg. Chem. 24, 509–514.
LinkOut - more resources
Full Text Sources
Miscellaneous