4-(2,3-Di-chloro-phen-yl)piperazin-1-ium picrate
- PMID: 36339106
- PMCID: PMC9462331
- DOI: 10.1107/S2414314621003795
4-(2,3-Di-chloro-phen-yl)piperazin-1-ium picrate
Abstract
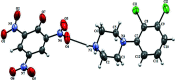
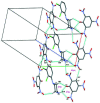
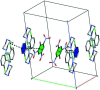
The title compound, C6H2N3O7 -·C10H13Cl2N2 +, crystallizes with one 1-(2,3-di-chloro-phen-yl)piperazine (DP) cation and one picrate (PA) anion in the asymmetric unit. In the crystal structure, the DP cation and PA anion are inter-connected via several N-H⋯O and C-H⋯O hydrogen bonds. The DP cation and PA anion are further connected through C-Cl⋯π [3.8201 (4), 3.7785 (4) Å] and N-O⋯π [3.7814 (4) Å] inter-actions. The DP cations are further inter-connected via a weak inter-molecular Cl⋯Cl [3.2613 (4) Å] halogen-halogen inter-action. The combination of these supra-molecular inter-actions leads to a herringbone like supra-molecular architecture.
Keywords: 1-(2,3-dichlorophenyl)piperazinium picrate; crystal structure; supramolecular interaction.
© Sathya et al. 2021.
Figures

References
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N. L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
-
- Braun, D. E., Gelbrich, T., Kahlenberg, V., Tessadri, R., Wieser, J. & Griesser, U. J. (2009). J. Pharm. Sci. 98, 2010–2026. - PubMed
-
- Bruker (2009). APEX2, SADABS and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cason, C. J. (2004). POV-RAY for Windows. Persistence of Vision, Raytracer Pty. Ltd, Victoria, Australia. URL: http://www.povray. org.
-
- Frank, H. M., GuoJun, Z. & QiPeng, Y. (2007). Journal of Beijing University of Chemical Technology, 34, 425–427.
LinkOut - more resources
Full Text Sources
