Rare disease emerging as a global public health priority
- PMID: 36339196
- PMCID: PMC9632971
- DOI: 10.3389/fpubh.2022.1028545
Rare disease emerging as a global public health priority
Abstract
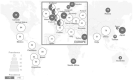
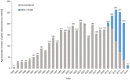
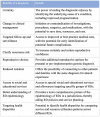
The genomics revolution over the past three decades has led to great strides in rare disease (RD) research, which presents a major shift in global policy landscape. While RDs are individually rare, there are common challenges and unmet medical and social needs experienced by the RD population globally. The various disabilities arising from RDs as well as diagnostic and treatment uncertainty were demonstrated to have detrimental influence on the health, psychosocial, and economic aspects of RD families. Despite the collective large number of patients and families affected by RDs internationally, the general lack of public awareness and expertise constraints have neglected and marginalized the RD population in health systems and in health- and social-care policies. The current Coronavirus Disease of 2019 (COVID-19) pandemic has exposed the long-standing and fundamental challenges of the RD population, and has reminded us of the critical need of addressing the systemic inequalities and widespread disparities across populations and jurisdictions. Owing to the commonality in goals between RD movements and universal health coverage targets, the United Nations (UN) has highlighted the importance of recognizing RDs in policies, and has recently adopted the UN Resolution to promote greater integration of RDs in the UN agenda, advancing UN's commitment in achieving the 2030 Sustainable Development Goals of "leav[ing] no one behind." Governments have also started to launch Genome Projects in their respective jurisdictions, aiming to integrate genomic medicine into mainstream healthcare. In this paper, we review the challenges experienced by the RD population, the establishment and adoption of RD policies, and the state of evidence in addressing these challenges from a global perspective. The Hong Kong Genome Project was illustrated as a case study to highlight the role of Genome Projects in enhancing clinical application of genomic medicine for personalized medicine and in improving equity of access and return in global genomics. Through reviewing what has been achieved to date, this paper will provide future directions as RD emerges as a global public health priority, in hopes of moving a step toward a more equitable and inclusive community for the RD population in times of pandemics and beyond.
Keywords: Hong Kong Genome Project; diversity; genomic equity; inclusiveness; public health priority; rare disease.
Copyright © 2022 Chung, Hong Kong Genome Project, Chu and Chung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Orphanet . The Portal for Rare Diseases and Orphan Drugs 2012. Available online at: https://www.orpha.net/consor/cgi-bin/Education_AboutRareDiseases.php?lng=EN (accessed May 20, 2022).
-
- Gopal-Srivastava R, Kaufmann P. Facilitating clinical studies in rare diseases. In:Posada de la Paz M, Taruscio D, Groft SC, editors. Rare Diseases Epidemiology: Update and Overview. Cham: Springer International Publishing. (2017). p. 125–40. - PubMed
-
- European Union . Regulation (EC) N°141/2000 of the European Parliament of the Council of 16 December 1999 on Orphan Medicinal Products. (2000). Available online at: https://eur-lex.europa.eu/EN/legal-content/summary/medicines-for-rare-di... (accessed May 20, 2022).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

