A binocular perception deficit characterizes prey pursuit in developing mice
- PMID: 36339264
- PMCID: PMC9626674
- DOI: 10.1016/j.isci.2022.105368
A binocular perception deficit characterizes prey pursuit in developing mice
Abstract
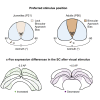
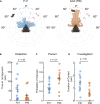
Integration of binocular information at the cellular level has long been studied in the mouse model to uncover the fundamental developmental mechanisms underlying mammalian vision. However, we lack an understanding of the corresponding ontogeny of visual behavior in mice that relies on binocular integration. To address this major outstanding question, we quantified the natural visually guided behavior of postnatal day 21 (P21) and adult mice using a live prey capture assay and a computerized-spontaneous perception of objects task (C-SPOT). We found a robust and specific binocular visual field processing deficit in P21 mice as compared to adults that corresponded to a selective increase in c-Fos expression in the anterior superior colliculus (SC) of the juveniles after C-SPOT. These data link a specific binocular perception deficit in developing mice to activity changes in the SC.
Keywords: Biological sciences; Neuroscience; Sensory neuroscience.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hensch T.K. Critical period regulation. Annu. Rev. Neurosci. 2004;27:549–579. - PubMed
-
- Cang J., Savier E., Barchini J., Liu X. Visual function, organization, and development of the mouse superior colliculus. Annu. Rev. Vis. Sci. 2018;4:239–262. - PubMed
-
- Hooks B.M., Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–326. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

