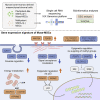
Single-cell RNA sequencing reveals different signatures of mesenchymal stromal cell pluripotent-like and multipotent populations
- PMID: 36339265
- PMCID: PMC9633745
- DOI: 10.1016/j.isci.2022.105395
Single-cell RNA sequencing reveals different signatures of mesenchymal stromal cell pluripotent-like and multipotent populations
Abstract
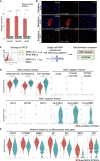
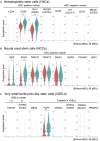
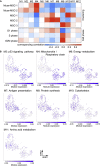
Somatic stem cells are advantageous research targets for understanding the properties required to maintain stemness. Human bone marrow-mesenchymal stromal cells (BM-MSCs) were separated into pluripotent-like SSEA-3(+) Muse cells (Muse-MSCs) and multipotent SSEA-3(-) MSCs (MSCs) and were subjected to single-cell RNA sequencing analysis. Compared with MSCs, Muse-MSCs exhibited higher expression levels of the p53 repressor MDM2; signal acceptance-related genes EGF, VEGF, PDGF, WNT, TGFB, INHB, and CSF; ribosomal protein; and glycolysis and oxidative phosphorylation. Conversely, MSCs had higher expression levels of FGF and ANGPT; Rho family and caveola-related genes; amino acid and cofactor metabolism; MHC class I/II, and lysosomal enzyme genes than Muse-MSCs. Unsupervised clustering further divided Muse-MSCs into two clusters stratified by the expression of cell cycle-related genes, and MSCs into three clusters stratified by the expression of cell cycle-, cytoskeleton-, and extracellular matrix-related genes. This study evaluating the differentiation ability of BM-MSC subpopulations provides intriguing insights for understanding stemness.
Keywords: Biological sciences; Cell biology; Omics; Stem cells research; Systems biology; Transcriptomics.
© 2022 The Author(s).
Conflict of interest statement
S. Wakao, Y. Kushida, Y. Kuroda, and M. Dezawa are parties to a co-development agreement with Life Science Institute, Inc. (LSII; Tokyo, Japan). S. Wakao and M. Dezawa have a patent for Muse cells, and the isolation method thereof is licensed to LSII.
Figures



Similar articles
-
The secretome of MUSE cells contains factors that may play a role in regulation of stemness, apoptosis and immunomodulation.Cell Cycle. 2017 Jan 2;16(1):33-44. doi: 10.1080/15384101.2016.1211215. Epub 2016 Jul 27. Cell Cycle. 2017. PMID: 27463232 Free PMC article.
-
Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton's jelly and bone marrow-derived mesenchymal stem cells.Stem Cell Res Ther. 2017 Apr 26;8(1):102. doi: 10.1186/s13287-017-0555-9. Stem Cell Res Ther. 2017. PMID: 28446235 Free PMC article.
-
Multilineage-differentiating stress-enduring (Muse)-like cells exist in synovial tissue.Regen Ther. 2018 Nov 20;10:17-26. doi: 10.1016/j.reth.2018.10.005. eCollection 2019 Jun. Regen Ther. 2018. PMID: 30525067 Free PMC article.
-
The Muse Cell Discovery, Thanks to Wine and Science.Adv Exp Med Biol. 2018;1103:1-11. doi: 10.1007/978-4-431-56847-6_1. Adv Exp Med Biol. 2018. PMID: 30484221 Review.
-
Muse Cells Provide the Pluripotency of Mesenchymal Stem Cells: Direct Contribution of Muse Cells to Tissue Regeneration.Cell Transplant. 2016;25(5):849-61. doi: 10.3727/096368916X690881. Epub 2016 Feb 15. Cell Transplant. 2016. PMID: 26884346 Review.
Cited by
-
Biological characteristics of Muse cells derived from MenSCs and their application in acute liver injury and intracerebral hemorrhage diseases.Regen Ther. 2024 Mar 10;27:48-62. doi: 10.1016/j.reth.2024.03.003. eCollection 2024 Dec. Regen Ther. 2024. PMID: 38496012 Free PMC article.
-
Functional variation among mesenchymal stem cells derived from different tissue sources.PeerJ. 2024 Jun 28;12:e17616. doi: 10.7717/peerj.17616. eCollection 2024. PeerJ. 2024. PMID: 38952966 Free PMC article.
-
Multipotent/pluripotent stem cell populations in stromal tissues and peripheral blood: exploring diversity, potential, and therapeutic applications.Stem Cell Res Ther. 2024 May 12;15(1):139. doi: 10.1186/s13287-024-03752-x. Stem Cell Res Ther. 2024. PMID: 38735988 Free PMC article. Review.
-
The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy.Stem Cell Res Ther. 2023 Dec 20;14(1):381. doi: 10.1186/s13287-023-03587-y. Stem Cell Res Ther. 2023. PMID: 38124129 Free PMC article. Review.
-
Stem cell therapy: A promising therapeutic approach for skeletal muscle atrophy.World J Stem Cells. 2025 Feb 26;17(2):98693. doi: 10.4252/wjsc.v17.i2.98693. World J Stem Cells. 2025. PMID: 40061264 Free PMC article. Review.
References
-
- Abe T., Aburakawa D., Niizuma K., Iwabuchi N., Kajitani T., Wakao S., Kushida Y., Dezawa M., Borlongan C.V., Tominaga T. Intravenously transplanted human multilineage-differentiating stress-enduring cells afford brain repair in a mouse lacunar stroke model. Stroke. 2020;51:601–611. doi: 10.1161/STROKEAHA.119.026589. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

