Clonorchis sinensis infection induces hepatobiliary injury via disturbing sphingolipid metabolism and activating sphingosine 1-phosphate receptor 2
- PMID: 36339341
- PMCID: PMC9627039
- DOI: 10.3389/fcimb.2022.1011378
Clonorchis sinensis infection induces hepatobiliary injury via disturbing sphingolipid metabolism and activating sphingosine 1-phosphate receptor 2
Abstract
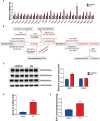
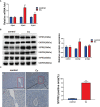
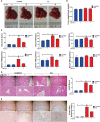
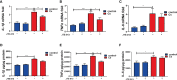
Clonorchis sinensis (C. sinensis) infection induces severe hepatobiliary injuries, which can cause inflammation, periductal fibrosis, and even cholangiocarcinoma. Sphingolipid metabolic pathways responsible for the generation of sphingosine-1-phosphate (S1P) and its receptor S1P receptors (S1PRs) have been implicated in many liver-related diseases. However, the role of S1PRs in C. sinensis-mediated biliary epithelial cells (BECs) proliferation and hepatobiliary injury has not been elucidated. In the present study, we found that C. sinensis infection resulted in alteration of bioactive lipids and sphingolipid metabolic pathways in mice liver. Furthermore, S1PR2 was predominantly activated among these S1PRs in BECs both in vivo and in vitro. Using JTE-013, a specific antagonist of S1PR2, we found that the hepatobiliary pathological injuries, inflammation, bile duct hyperplasia, and periductal fibrosis can be significantly inhibited in C. sinensis-infected mice. In addition, both C. sinensis excretory-secretory products (CsESPs)- and S1P-induced activation of AKT and ERK1/2 were inhibited by JTE-013 in BECs. Therefore, the sphingolipid metabolism pathway and S1PR2 play an important role, and may serve as potential therapeutic targets in hepatobiliary injury caused by C. sinensis-infection.
Keywords: Clonorchis sinensis; biliary epithelial cells (BECs); hepatobiliary injuries; sphingolipid metabolism; sphingosine 1-phosphate receptor 2.
Copyright © 2022 Liu, Liu, Yu, Zhao, Wang, Sun, Koda, Zhang, Yu, Yan, Tang, Jiang and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

