How Human TET2 Enzyme Catalyzes the Oxidation of Unnatural Cytosine Modifications in Double-Stranded DNA
- PMID: 36339349
- PMCID: PMC9629818
- DOI: 10.1021/acscatal.2c00024
How Human TET2 Enzyme Catalyzes the Oxidation of Unnatural Cytosine Modifications in Double-Stranded DNA
Abstract
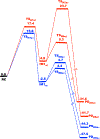
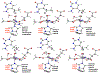
Methylation of cytosine bases is strongly linked to gene expression, imprinting, aging, and carcinogenesis. The Ten-eleven translocation (TET) family of enzymes, which are Fe(II)/2-oxoglutarate (2OG)-dependent enzymes, employ Fe(IV)=O species to dealkylate the lesioned bases to an unmodified cytosine. Recently, it has been shown that the TET2 enzyme can catalyze promiscuously DNA substrates containing unnatural alkylated cytosine. Such unnatural substrates of TET can be used as direct probes for measuring the TET activity or capturing TET from cellular samples. Herein, we studied the catalytic mechanisms during the oxidation of the unnatural C5-position modifications (5-ethylcytosine (5eC), 5-vinylcytosine (5vC) and 5-ethynylcytosine (5eyC)) and the demethylation of N4-methylated lesions (4-methylcytosine (4mC) and 4,4-dimethylcytosine(4dmC)) of the cytosine base by the TET2 enzyme using molecular dynamics (MD) and combined quantum mechanics and molecular mechanics (QM/MM) computational approaches. The results reveal that the chemical nature of the alkylation of the double-stranded (ds) DNA substrates induces distinct changes in the interactions in the binding site, the second coordination sphere, and long-range correlated motions of the ES complexes. The rate-determining hydrogen atom transfer (HAT) is faster in N4-methyl substituent substrates than in the C5-alkylations. Importantly, the calculations show the preference of hydroxylation over desaturation in both 5eC and 5vC substrates. The studies elucidate the post-hydroxylation rearrangements of the hydroxylated intermediates of 5eyC and 5vC to ketene and 5-formylmethylcytosine (5fmC), respectively, and hydrolysis of hemiaminal intermediate of 4mC to formaldehyde and unmodified cytosine proceed exclusively in aqueous solution outside of the enzyme environment. Overall, the studies show that the chemical nature of the unnatural alkylated cytosine substrates exercises distinct effects on the binding interactions, reaction mechanism, and dynamics of TET2.
Keywords: DNA repair; QM/MM calculations; TET2 enzyme; demethylation; molecular dynamics; non-heme enzymes; reaction mechanism.
Figures
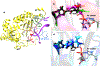
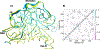
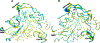
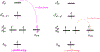
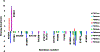





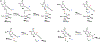
References
-
- Mahfoudhi E; Talhaoui I; Cabagnols X; Della Valle V; Secardin L; Rameau P; Bernard OA; Ishchenko AA; Abbes S; Vainchenker W; Saparbaev M; Plo I TET2-Mediated 5-Hydroxymethylcytosine Induces Genetic Instability and Mutagenesis. DNA Repair 2016, 43, 78–88. - PubMed
-
- Bird A DNA Methylation Patterns and Epigenetic Memory. Genes Dev. 2002, 16 (1), 6–21. - PubMed
-
- Branco MR; Ficz G; Reik W Uncovering the Role of 5-Hydroxymethylcytosine in the Epigenome. Nat. Rev. Genet. 2012, 13 (1), 7–13. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
