Genetic conditions of short stature: A review of three classic examples
- PMID: 36339399
- PMCID: PMC9634554
- DOI: 10.3389/fendo.2022.1011960
Genetic conditions of short stature: A review of three classic examples
Abstract
Noonan, Turner, and Prader-Willi syndromes are classical genetic disorders that are marked by short stature. Each disorder has been recognized for several decades and is backed by extensive published literature describing its features, genetic origins, and optimal treatment strategies. These disorders are accompanied by a multitude of comorbidities, including cardiovascular issues, endocrinopathies, and infertility. Diagnostic delays, syndrome-associated comorbidities, and inefficient communication among the members of a patient's health care team can affect a patient's well-being from birth through adulthood. Insufficient information is available to help patients and their multidisciplinary team of providers transition from pediatric to adult health care systems. The aim of this review is to summarize the clinical features and genetics associated with each syndrome, describe best practices for diagnosis and treatment, and emphasize the importance of multidisciplinary teams and appropriate care plans for the pediatric to adult health care transition.
Keywords: Noonan syndrome; Prader-Willi syndrome; Turner syndrome; genetics; growth hormone; short stature.
Copyright © 2022 Butler, Miller, Romano, Ross, Abuzzahab, Backeljauw, Bamba, Bhangoo, Mauras and Geffner.
Conflict of interest statement
AR is a speaker and consultant for Novo Nordisk and a consultant for Ascendis Pharma. JR is a consultant and has research support from Novo Nordisk and is a consultant with OPKO. MA receives research support from Ascendis, Levo, Lumos, Novo Nordisk, Rhythm, and Soleno. MA has been on advisory boards for Pfizer and Rhythm. PB has been a consultant for Novo Nordisk, Novartis/Sandoz, Tolmar, Ascendis Pharma, BioMarin, Cavalry Bioventures, and Ipsen, and currently receives research support from Novo Nordisk and Ipsen. NM has received institutional research grants from Novo Nordisk and OPKO and has received consulting fees from Agios. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
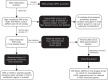
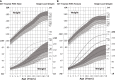
Figures

References
-
- Allen MJ, Sharma S. Noonan syndrome. In: StatPearls. FL: Treasure Island; (2021).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

