Leptin receptor antagonist attenuates experimental autoimmune thyroiditis in mice by regulating Treg/Th17 cell differentiation
- PMID: 36339447
- PMCID: PMC9630560
- DOI: 10.3389/fendo.2022.1042511
Leptin receptor antagonist attenuates experimental autoimmune thyroiditis in mice by regulating Treg/Th17 cell differentiation
Abstract
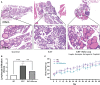
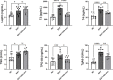
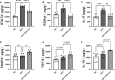
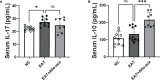
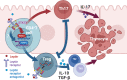
Leptin has been found to be involved in the development and progression of many autoimmune diseases. As an organ-specific autoimmune disease, the pathogenesis of Hashimoto's thyroiditis has not been fully elucidated. It has been reported that serum leptin level is increased in Hashimoto's thyroiditis, but other studies have not shown any difference. We replicated a mouse model of experimental autoimmune thyroiditis (EAT) with a high-iodine diet and found that injection of the leptin receptor antagonist Allo-aca reduced thyroid follicle destruction and inflammatory cell infiltration in EAT mice, and thyroxine and thyroid autoimmune antibody levels. Further investigation revealed that Allo-aca promotes the differentiation of Treg cells and inhibits the differentiation of Th17 cells. We believe that Allo-aca can alter the differentiation of Treg/Th17 cells by inhibiting the leptin signaling pathway, thereby alleviating thyroid injury in EAT mice. Interfering with the leptin signaling pathway may be a novel new approach to treat treating and ameliorating Hashimoto's thyroiditis.
Keywords: JAK2/STAT3 pathway; NOD/ShiLtJ mouse; experimental autoimmune thyroiditis (EAT); leptin; leptin receptor antagonist.
Copyright © 2022 Wang, Zhang, Jiang, Zhao, Xu, Zeng, Xu and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

