4-Amino-6-(piperidin-1-yl)pyrimidine-5-carbo-nitrile
- PMID: 36339485
- PMCID: PMC9462203
- DOI: 10.1107/S2414314620003855
4-Amino-6-(piperidin-1-yl)pyrimidine-5-carbo-nitrile
Abstract
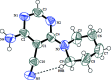
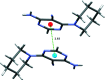
In the title compound, C10H13N5, the piperidine ring adopts a chair conformation with the exocyclic N-C bond in an axial orientation, and the dihedral angle between the mean planes of piperidine and pyrimidine rings is 49.57 (11)°. A short intra-molecular C-H⋯N contact generates an S(7) ring. In the crystal, N-H⋯N hydrogen bonds link the mol-ecules into (100) sheets and a weak aromatic π-π stacking inter-action is observed [centroid-centroid separation = 3.5559 (11) Å] between inversion-related pyrimidine rings.
Keywords: N—H⋯N hydrogen bonds; crystal structure; pyrimidine.
© Bhat et al. 2020.
Figures

References
-
- Bruker. (1998). SMART, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Fang, Y., Xiong, L., Hu, J., Zhang, S., Xie, S., Tu, L., Wan, Y., Jin, Y., Li, X., Hu, S. & Yang, Z. (2019). Bioorg. Chem. 86, 103–111. - PubMed
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
-
- Hassan, A. S., Mady, M. F., Awad, H. M. & Hafez, T. S. (2017). Chin. Chem. Lett. 28, 388–393.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
