Real-world study of antiresorptive-related osteonecrosis of jaw based on the US food and drug administration adverse event reporting system database
- PMID: 36339548
- PMCID: PMC9627332
- DOI: 10.3389/fphar.2022.1017391
Real-world study of antiresorptive-related osteonecrosis of jaw based on the US food and drug administration adverse event reporting system database
Abstract
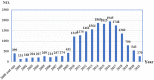
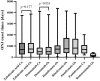
Objective: This study aims to explore the risk signals of osteonecrosis of the jaw induced by antiresorptive drugs and provide references for the clinical safety application. Method: According to the FDA's Adverse Event Reporting System (FAERS), from January 2004 to September 2021, we chose "Osteonecrosis of the jaw (10064658)" and "Exposed bone in jaw (10071014)" as preferred terms, "antiresorptive drugs" as the target drugs, and primary suspect drug as the drug role code in the dataset. We evaluated the association between drugs and adverse events by using reporting odds ratio (ROR) based on disproportionality analysis. We took the High-Level Terms (HLT) of MedDRA® as the classification level of indications to calculate ROR to compare the signal difference of ONJ in different indications. In addition, patients with antiresorptive-induced osteonecrosis of the jaw and the time of onset of the condition following different antiresorptive medications were collected for the study. Results: The FAERS contained 18,421 reports relating to jaw osteonecrosis from January 2004 to September 2021. A total of eight antiresorptive agents were included in the analysis. From high to low, the ROR of ONJ induced by antiresorptive agents (regardless of indication) is pamidronate (ROR = 494.8), zoledronic acid (ROR = 431.9), denosumab (ROR = 194.8), alendronate (ROR = 151.2), risedronate (ROR = 140.2), etidronic acid (ROR = 64.5), ibandronate (ROR = 40.8), and romosozumab (ROR = 6.4). HLT ROR values for "metabolic bone disorders" were the lowest for each drug, while HLT ROR values were high for "tumor-related indications," including breast and nipple neoplasms malignant, plasma cell myelomas, and prostatic neoplasms malignant. The onset time for osteonecrosis of the jaw as median (Q1, Q3), osteoporosis-related indications, and the onset time for ONJ were 730 (368, 1268), 489.5 (236.3, 909.8), 722.5 (314, 1055), 761 (368, 1720), and 153 (50, 346) for zoledronic acid, denosumab, ibandronate, risedronate, and romosozumab, respectively. Cancer-related indications: the onset time for ONJ were 680.5 (255.3, 1283), 488 (245, 851), and 696.5 (347, 1087) for zoledronic acid, denosumab, and pamidronate, respectively. Conclusion: When antiresorptive drugs are used for metastasis, they have the largest risk signal, followed by malignancy, and the smallest is osteoporosis. The onset time of ONJ may not be related to the indications. The onset time of ONJ for BPs was about 2 years, denosumab about 1.3 years, and romosozumab less than 1 year, which may be related to sequential treatment. When used according to the instructions, the risk of ONJ caused by denosumab was higher than that of zoledronic acid, regardless of the indication. Based on these findings, researchers will continue to monitor and identify risk factors.
Keywords: antiresorptive drugs; database; osteonecrosis of jaw; romosozumab; signal processing; the US food and drug administration adverse event reporting system.
Copyright © 2022 Peng, Wang, Liu, Xu, Wang, Chen, Wu, Ren, Liang, Liu and Ban.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Anastasilakis A. D., Pepe J., Napoli N., Palermo A., Magopoulos C., Khan A. A., et al. (2022). Osteonecrosis of the jaw and antiresorptive agents in benign and malignant diseases: A critical review organized by the ects. J. Clin. Endocrinol. Metab. 107 (5), 1441–1460. 10.1210/clinem/dgab888 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

