Adenosine monophosphate activated protein kinase contributes to skeletal muscle health through the control of mitochondrial function
- PMID: 36339617
- PMCID: PMC9632297
- DOI: 10.3389/fphar.2022.947387
Adenosine monophosphate activated protein kinase contributes to skeletal muscle health through the control of mitochondrial function
Abstract
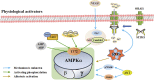
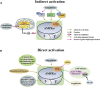
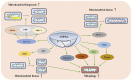
Skeletal muscle is one of the largest organs in the body and the largest protein repository. Mitochondria are the main energy-producing organelles in cells and play an important role in skeletal muscle health and function. They participate in several biological processes related to skeletal muscle metabolism, growth, and regeneration. Adenosine monophosphate-activated protein kinase (AMPK) is a metabolic sensor and regulator of systemic energy balance. AMPK is involved in the control of energy metabolism by regulating many downstream targets. In this review, we propose that AMPK directly controls several facets of mitochondrial function, which in turn controls skeletal muscle metabolism and health. This review is divided into four parts. First, we summarize the properties of AMPK signal transduction and its upstream activators. Second, we discuss the role of mitochondria in myogenesis, muscle atrophy, regeneration post-injury of skeletal muscle cells. Third, we elaborate the effects of AMPK on mitochondrial biogenesis, fusion, fission and mitochondrial autophagy, and discuss how AMPK regulates the metabolism of skeletal muscle by regulating mitochondrial function. Finally, we discuss the effects of AMPK activators on muscle disease status. This review thus represents a foundation for understanding this biological process of mitochondrial dynamics regulated by AMPK in the metabolism of skeletal muscle. A better understanding of the role of AMPK on mitochondrial dynamic is essential to improve mitochondrial function, and hence promote skeletal muscle health and function.
Keywords: AMPK; mitochondria; muscle atrophy; muscle regeneration; skeletal muscle.
Copyright © 2022 Yan, Li, Lin, Ji, Wang, Yan, Shen, Wang, Huang, Jiang, Sun and Qi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

