Ethyl 10α-hy-droxy-4,9-dimethyl-14-oxo-3,8,15-trioxa-tetra-cyclo-[10.3.0.02,4.07,9]penta-decane-13-spiro-5'-pyrazole-3'-carboxyl-ate
- PMID: 36339783
- PMCID: PMC9462250
- DOI: 10.1107/S2414314620009451
Ethyl 10α-hy-droxy-4,9-dimethyl-14-oxo-3,8,15-trioxa-tetra-cyclo-[10.3.0.02,4.07,9]penta-decane-13-spiro-5'-pyrazole-3'-carboxyl-ate
Abstract
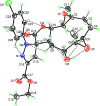
The ten-membered ring in the title mol-ecule, C25H29ClN2O7, adopts an approximate chair-chair conformation, whereas the five-membered furan and pyrazole rings display envelope conformations. The mean plane of the furan ring is almost perpendicular to that of the pyrazole ring, as indicated by the dihedral angle between them of 86.45 (9)°. The pyrazole ring is slightly inclined to the plane of the attached phenyl ring, subtending a dihedral angle of 16.88 (8)°. The conformation of the mol-ecule is stabilized by six intra-molecular hydrogen bonds and crystal cohesion is ensured by five C-H⋯O hydrogen bonds, in addition to C-H⋯π inter-actions.
Keywords: crystal structure; furan; hydrogen bonding; pyrazole.
© Outahar et al. 2020.
Figures

References
-
- Abdel Sattar, E., Galal, A. M. & Mossa, J. S. (1996). J. Nat. Prod. 59, 403–405. - PubMed
-
- Bellakhdar, J. (1997). La Pharmacopée Marocaine Traditionnelle, pp. 272–274. Paris: Edition Ibis Press.
-
- Bruker (2016). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- El Hassany, B., El Hanbali, F., Akssira, M., Mellouki, F., Haidour, A. & Barrero, A. F. (2004). Fitoterapia, 75, 573–576. - PubMed
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
LinkOut - more resources
Full Text Sources
Research Materials
