1-(2-Methyl-phen-yl)-4,4'-bipyridin-1-ium tetra-fluorido-borate
- PMID: 36339800
- PMCID: PMC9462016
- DOI: 10.1107/S2414314622002486
1-(2-Methyl-phen-yl)-4,4'-bipyridin-1-ium tetra-fluorido-borate
Abstract
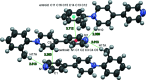
Crystals of the title compound, C17H15N2 +·BF4 -, were unexpectedly grown from crystallization attempts of [Pt(4,4'-bpy)4](BF4)2 [Smith et al. (2019 ▸). Comments Inorg. Chem. 39, 188-215] using toluene and aceto-nitrile. The tetra-fluoro-borate anion and the central pyridinium ring of the cation are disordered, with atomic site occupancies close to ½. The tolyl group of the cation has a 75.31 (11)° twist relative to the unsubstituted pyridyl group. This rotation allows for a centrosymmetric dimer of cations with weak hydrogen bonding between the pyridyl nitro-gen atom and a methyl H atom on the neighbouring cation.
Keywords: bipyridinium; crystal structure; hydrogen bond.
© Welton et al. 2022.
Figures




References
-
- Coe, B. J., Harris, J. A., Harrington, L. J., Jeffery, J. C., Rees, L. H., Houbrechts, S. & Persoons, A. (1998). Inorg. Chem. 37, 3391–3399.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Lin, F. & Zhao, X. (2015). Tetrahedron, 71, 1124–1131.
-
- Rigaku OD (2019). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
LinkOut - more resources
Full Text Sources
Miscellaneous
