Electronic cigarettes versus nicotine-replacement therapy for smoking cessation: A systematic review and meta-analysis of randomized controlled trials
- PMID: 36339933
- PMCID: PMC9582581
- DOI: 10.18332/tid/154075
Electronic cigarettes versus nicotine-replacement therapy for smoking cessation: A systematic review and meta-analysis of randomized controlled trials
Abstract
Introduction: Nicotine-replacement therapy (NRT) and electronic cigarettes (e-cigarettes) have been frequently used for smoking cessation. The aim of this review is to investigate the effectiveness and safety of e-cigarettes versus NRT for smoking cessation.
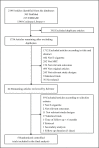
Methods: We searched PubMed, EMBASE, the Cochrane Library from inception to 10 October 2021. We included randomized controlled trials (RCTs) comparing e-cigarettes versus NRT for smoking cessation. Two authors independently screened titles, abstracts and full texts for eligibility. Paired authors extracted data, assessed risk of bias, and used GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) to rate the certainty of evidence.
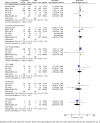
Results: The study included five RCTs with 1748 participants. The meta-analysis suggested the e-cigarettes versus NRT increased the ≥6 months continuous abstinence rate (RR=1.67; 95% CI: 1.21-2.28; 55 more per 1000 participants, low certainty), and 7-day point abstinence rate at ≥6 months follow-up (RR=1.43; 95% CI: 1.19-1.72; 84 more per 1000, low certainty). However, we found no evidence that e-cigarettes versus NRT increased 3-6 months continuous abstinence rate (RR=1.07; 95% CI: 0.73-1.57; 10 more per 1000, very low certainty) and <3 months continuous abstinence rate (RR=1.20; 95% CI: 0.90-1.60; 54 more per 1000, low certainty); similar results were found at <3 months follow-up (RR=1.19; 95% CI: 0.92-1.54; 55 more per 1000, very low certainty) and 3-6 months follow-up in 7-day point abstinence rate (RR=1.01; 95% CI: 0.70-1.44; 2 more per 1000, very low certainty). The adverse events were not significant between e-cigarettes and NRT other than throat irritation (RR=1.27; 95% CI: 1.13-1.42; 118 more per 1000, low certainty).
Conclusions: E-cigarettes appeared to be superior to NRT in ≥6 months continuous abstinence rate and 7-day point abstinence rate. At short-term duration, we found no evidence that e-cigarettes compared to NRT increased the <6 months continuous abstinence rate and 7-day point abstinence rate.
Keywords: 7-day point abstinence rate; continuous abstinence rate; electronic cigarettes; nicotine-replacement therapy.
© 2022 Li J. et al.
Conflict of interest statement
The authors have each completed and submitted an ICMJE form for disclosure of potential conflicts of interest. The authors declare that they have no competing interests, financial or otherwise, related to the current work. All the authors report that since the initial planning of the work that this study was supported by the Major Project of the National Social Science Fund of China: Research on the Theoretical System, International Experience, and Chinese Path of Evidence-based Social Science (No. 19ZDA14).
Figures



References
-
- World Health Organization Tobacco. Accessed March 9, 2022. https://www.who.int/health-topics/tobacco#tab=tab_2.
Publication types
LinkOut - more resources
Full Text Sources
