Pathological implications of metabolic reprogramming and its therapeutic potential in medulloblastoma
- PMID: 36340043
- PMCID: PMC9627342
- DOI: 10.3389/fcell.2022.1007641
Pathological implications of metabolic reprogramming and its therapeutic potential in medulloblastoma
Abstract
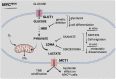
Tumor-specific alterations in metabolism have been recognized to sustain the production of ATP and macromolecules needed for cell growth, division and survival in many cancer types. However, metabolic heterogeneity poses a challenge for the establishment of effective anticancer therapies that exploit metabolic vulnerabilities. Medulloblastoma (MB) is one of the most heterogeneous malignant pediatric brain tumors, divided into four molecular subgroups (Wingless, Sonic Hedgehog, Group 3 and Group 4). Recent progresses in genomics, single-cell sequencing, and novel tumor models have updated the classification and stratification of MB, highlighting the complex intratumoral cellular diversity of this cancer. In this review, we emphasize the mechanisms through which MB cells rewire their metabolism and energy production networks to support and empower rapid growth, survival under stressful conditions, invasion, metastasis, and resistance to therapy. Additionally, we discuss the potential clinical benefits of currently available drugs that could target energy metabolism to suppress MB progression and increase the efficacy of the current MB therapies.
Keywords: OXPHOS (oxidative phosphorylation); ROS; glutamine/glutamate (GABA) cycle; metabolism; warburg effect.
Copyright © 2022 Marabitti, Giansanti, De Mitri, Gatto, Mastronuzzi and Nazio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Novel Molecular Hallmarks of Group 3 Medulloblastoma by Single-Cell Transcriptomics.Front Oncol. 2021 Mar 18;11:622430. doi: 10.3389/fonc.2021.622430. eCollection 2021. Front Oncol. 2021. PMID: 33816256 Free PMC article.
-
Methylation Profiling of Medulloblastoma in a Clinical Setting Permits Sub-classification and Reveals New Outcome Predictions.Front Neurol. 2020 Mar 20;11:167. doi: 10.3389/fneur.2020.00167. eCollection 2020. Front Neurol. 2020. PMID: 32265819 Free PMC article.
-
Medulloblastoma: clinicopathological parameters, risk stratification, and survival analysis of immunohistochemically validated molecular subgroups.J Egypt Natl Canc Inst. 2021 Feb 8;33(1):6. doi: 10.1186/s43046-021-00060-w. J Egypt Natl Canc Inst. 2021. PMID: 33555447
-
Molecular Heterogeneity and Cellular Diversity: Implications for Precision Treatment in Medulloblastoma.Cancers (Basel). 2020 Mar 10;12(3):643. doi: 10.3390/cancers12030643. Cancers (Basel). 2020. PMID: 32164294 Free PMC article. Review.
-
Molecular Classification of Medulloblastoma.Neurol Med Chir (Tokyo). 2016 Nov 15;56(11):687-697. doi: 10.2176/nmc.ra.2016-0016. Epub 2016 May 26. Neurol Med Chir (Tokyo). 2016. PMID: 27238212 Free PMC article. Review.
Cited by
-
Deciphering Medulloblastoma: Epigenetic and Metabolic Changes Driving Tumorigenesis and Treatment Outcomes.Biomedicines. 2025 Aug 4;13(8):1898. doi: 10.3390/biomedicines13081898. Biomedicines. 2025. PMID: 40868156 Free PMC article. Review.
-
Isocitrate dehydrogenase 1 primes group-3 medulloblastomas for cuproptosis.Cancer Cell. 2025 Jun 9;43(6):1159-1174.e8. doi: 10.1016/j.ccell.2025.04.013. Epub 2025 May 15. Cancer Cell. 2025. PMID: 40378837
-
Overcoming Treatment Resistance in Medulloblastoma: Underlying Mechanisms and Potential Strategies.Cancers (Basel). 2024 Jun 18;16(12):2249. doi: 10.3390/cancers16122249. Cancers (Basel). 2024. PMID: 38927954 Free PMC article. Review.
-
Exploring the Molecular Complexity of Medulloblastoma: Implications for Diagnosis and Treatment.Diagnostics (Basel). 2023 Jul 18;13(14):2398. doi: 10.3390/diagnostics13142398. Diagnostics (Basel). 2023. PMID: 37510143 Free PMC article. Review.
-
Marinopyrrole derivative MP1 as a novel anti-cancer agent in group 3 MYC-amplified Medulloblastoma.J Exp Clin Cancer Res. 2024 Jan 11;43(1):18. doi: 10.1186/s13046-024-02944-w. J Exp Clin Cancer Res. 2024. PMID: 38200580 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

