Pyrazoline Containing Compounds as Therapeutic Targets for Neurodegenerative Disorders
- PMID: 36340076
- PMCID: PMC9631758
- DOI: 10.1021/acsomega.2c05339
Pyrazoline Containing Compounds as Therapeutic Targets for Neurodegenerative Disorders
Abstract
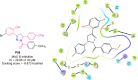
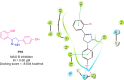
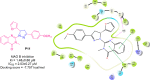
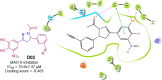
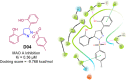
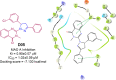
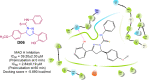
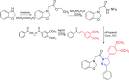
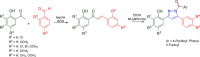
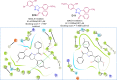
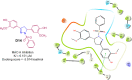
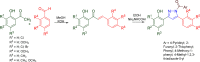
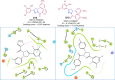
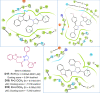
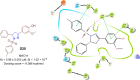
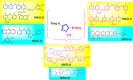
Pyrazolines are a significant class of heterocyclic compounds with essential biological activities. They are quite stable, which has inspired medicinal chemists to experiment with the ring's structure in many different ways to create a variety of pharmacological activities. The structures of numerous commercially available therapeutic agents contain a pyrazoline ring. Pyrazolines are well-known for their ability to treat neurodegenerative diseases. The neurodegenerative diseases that affect huge populations globally include Alzheimer's disease (AD), Parkinson's disease (PD), and psychiatric disorders. The neuroprotective properties of pyrazolines published since 2003 are covered in the current review. Structure-activity relationships (SARs), molecular docking simulation, anticholinesterase (anti-AChE), and monoamine oxidase (MAO A/B) inhibitory actions are all covered in this article. Pyrazolines were discovered to have beneficial effects in the management of AD and were revealed to be inhibitors of acetylcholine esterase (AChE) and beta-amyloid (Aβ) plaques. They were discovered to be efficient against PD and also targeted MAO B and COMT. It was discovered that the pyrazolines block MAO A to treat psychiatric diseases. Pyrazolines are significant heteroaromatic scaffolds with a variety of biological functions. They were discovered to be remarkably stable and serve as an indispensable anchor for the development of new drugs. By blocking AChE and MAOs, they may be used to treat neurodegenerative diseases. The discussion outlined here is an essential and helpful resource for medicinal chemists who are investigating and applying pyrazolines in neurodegenerative research initiatives as well as to expedite future research programs on neurodegenerative disorders.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


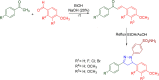

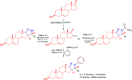








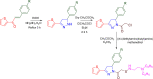





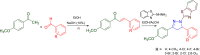


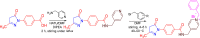





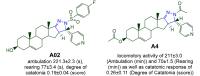
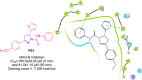

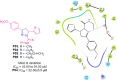




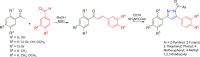













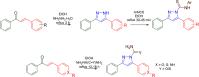


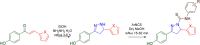
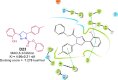


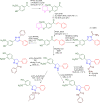
References
-
- Manna K.; Banik U.; Ghosh P.; Das M. A review on synthesis and pharmacological diversity of isoxazoles and pyrazolines. Pharmaceut. Sci. 2014, 1, 37–49.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

