N-terminal Domain of Amyloid-β Impacts Fibrillation and Neurotoxicity
- PMID: 36340079
- PMCID: PMC9631750
- DOI: 10.1021/acsomega.2c04583
N-terminal Domain of Amyloid-β Impacts Fibrillation and Neurotoxicity
Abstract
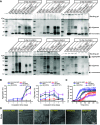
Alzheimer's disease is characterized by the presence of distinct amyloid-β peptide (Aβ) assemblies with diverse sizes, shapes, and toxicity. However, the primary determinants of Aβ aggregation and neurotoxicity remain unknown. Here, the N-terminal amino acid residues of Aβ42 that distinguished between humans and rats were substituted. The effects of these modifications on the ability of Aβ to aggregate and its neurotoxicity were investigated using biochemical, biophysical, and cellular techniques. The Aβ-derived diffusible ligand, protofibrils, and fibrils formed by the N-terminal mutational peptides, including Aβ42(R5G), Aβ42(Y10F), and rat Aβ42, were indistinguishable by conventional techniques such as size-exclusion chromatography, negative-staining transmission electron microscopy and silver staining, whereas the amyloid fibrillation detected by thioflavin T assay was greatly inhibited in vitro. Using circular dichroism spectroscopy, we discovered that both Aβ42 and Aβ42(Y10F) generated protofibrils and fibrils with a high proportion of parallel β-sheet structures. Furthermore, protofibrils formed by other mutant Aβ peptides and N-terminally shortened peptides were incapable of inducing neuronal death, with the exception of Aβ42 and Aβ42(Y10F). Our findings indicate that the N-terminus of Aβ is important for its fibrillation and neurotoxicity.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Analysis of the secondary structure of beta-amyloid (Abeta42) fibrils by systematic proline replacement.J Biol Chem. 2004 Dec 10;279(50):52781-8. doi: 10.1074/jbc.M406262200. Epub 2004 Sep 30. J Biol Chem. 2004. PMID: 15459202
-
The conformational epitope for a new Aβ42 protofibril-selective antibody partially overlaps with the peptide N-terminal region.J Neurochem. 2017 Dec;143(6):736-749. doi: 10.1111/jnc.14211. Epub 2017 Nov 22. J Neurochem. 2017. PMID: 28881033 Free PMC article.
-
Role of Species-Specific Primary Structure Differences in Aβ42 Assembly and Neurotoxicity.ACS Chem Neurosci. 2015 Dec 16;6(12):1941-55. doi: 10.1021/acschemneuro.5b00180. Epub 2015 Oct 19. ACS Chem Neurosci. 2015. PMID: 26421877 Free PMC article.
-
N-Terminal Extensions Retard Aβ42 Fibril Formation but Allow Cross-Seeding and Coaggregation with Aβ42.J Am Chem Soc. 2015 Nov 25;137(46):14673-85. doi: 10.1021/jacs.5b07849. Epub 2015 Nov 17. J Am Chem Soc. 2015. PMID: 26535489 Free PMC article.
-
Neurotoxicity and physicochemical properties of Abeta mutant peptides from cerebral amyloid angiopathy: implication for the pathogenesis of cerebral amyloid angiopathy and Alzheimer's disease.J Biol Chem. 2003 Nov 14;278(46):46179-87. doi: 10.1074/jbc.M301874200. Epub 2003 Aug 27. J Biol Chem. 2003. PMID: 12944403
Cited by
-
Sodium benzoate treatment decreased amyloid beta peptides and improved cognitive function among patients with Alzheimer's disease: secondary analysis of a randomized clinical trial.Transl Psychiatry. 2025 Aug 5;15(1):264. doi: 10.1038/s41398-025-03492-3. Transl Psychiatry. 2025. PMID: 40764372 Free PMC article. Clinical Trial.
-
Transient interactions between the fuzzy coat and the cross-β core of brain-derived Aβ42 filaments.Sci Adv. 2025 Jan 17;11(3):eadr7008. doi: 10.1126/sciadv.adr7008. Epub 2025 Jan 15. Sci Adv. 2025. PMID: 39813358 Free PMC article.
-
Neuroprotective Evaluation of Murraya Carbazoles: In Vitro and Docking Insights into Their Anti-AChE and Anti-Aβ Activities.Molecules. 2025 Jul 26;30(15):3138. doi: 10.3390/molecules30153138. Molecules. 2025. PMID: 40807313 Free PMC article.
-
Modulation of Alzheimer's Disease Aβ40 Fibril Polymorphism by the Small Heat Shock Protein αB-Crystallin.J Am Chem Soc. 2024 Jul 17;146(28):19077-19087. doi: 10.1021/jacs.4c03504. Epub 2024 Jul 7. J Am Chem Soc. 2024. PMID: 38973199 Free PMC article.
References
-
- Sevigny J.; Chiao P.; Bussière T.; Weinreb P. H.; Williams L.; Maier M.; Dunstan R.; Salloway S.; Chen T.; Ling Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. 10.1038/nature19323. - DOI - PubMed
- Sasaguri H.; Nilsson P.; Hashimoto S.; Nagata K.; Saito T.; De Strooper B.; Hardy J.; Vassar R.; Winblad B.; Saido T. C. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J 2017, 36, 2473.10.15252/embj.201797397. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

