Exploring Arylazo-3,5-Bis(trifluoromethyl)pyrazole Switches
- PMID: 36340122
- PMCID: PMC9631733
- DOI: 10.1021/acsomega.2c04984
Exploring Arylazo-3,5-Bis(trifluoromethyl)pyrazole Switches
Abstract
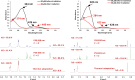
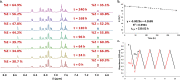
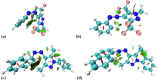
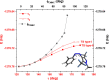
Arylazopyrazoles stand out among the azoheteroarene photoswitches due to their excellent properties in terms of stability of the least stable isomer and conversion between isomers, leading to their use in several interesting applications. We report herein the synthesis of arylazo-trifluoromethyl-substituted pyrazoles and their switching behavior under light irradiation. UV-vis and NMR experiments showed that arylazo-1H-3,5-bis(trifluoromethyl)pyrazoles displayed very long half-lives in DMSO (days), along with reasonable values of other parameters that characterize a photoswitch. Inclusion of naphthyl moieties as aryl counterparts of the arylazopyrazoles is beneficial only in combination with trifluoromethyl groups, while extending the conjugation by grafting the pyrazole moiety with electron-donating or -withdrawing substituents positively affects the photoswitching behavior, in terms of isomerization yield and half-lives of the least stable isomer. The experimental values were correlated with theoretical calculations indicating the valuable influence of the trifluoromethyl groups onto the photoswitching behavior.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
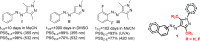








References
-
- Yang Z.; Liu Z.; Yuan L. Recent Advances of Photoresponsive Supramolecular Switches. Asian J. Org. Chem. 2021, 10 (1), 74–90. 10.1002/ajoc.202000501. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

