Are Nocebo Effects in Adulthood Linked to Prenatal Maternal Cortisol Levels?
- PMID: 36340278
- PMCID: PMC9597651
- DOI: 10.36131/cnfioritieditore20220505
Are Nocebo Effects in Adulthood Linked to Prenatal Maternal Cortisol Levels?
Abstract
Objective: Placebo-induced adverse events, or nocebo effects, occur when doctor-patient communication anticipates the onset of negative symptoms. They have been found to correlate with the anxiety-related activity of the hypothalamic-pituitary-adrenal system. Here we try to determine if prenatal hyperactivity of this system, as assessed through plasma cortisol, may influence nocebo effects in adulthood.
Method: We investigated the rate and magnitude of nocebo effects in 378 adults whose prenatal maternal plasma cortisol was measured during the first, second and third trimester of pregnancy. The healthy subjects underwent a nocebo oxygen challenge. This consisted of the inhalation of fake (placebo) oxygen and assessment of the following adverse events: headache, chest pain, abdominal pain, and cough. Plasma cortisol responses during the nocebo adverse events were also measured.
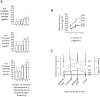
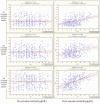
Results: 41 out of 46 (89.1%) subjects who reported 3 adverse events, and 37 out of 37 (100%) subjects who reported 4 adverse events had prenatal maternal cortisol above normal levels. By contrast, only 10 out of 143 (7%) subjects who reported 0 adverse events showed prenatal maternal cortisol above the normal range. Moreover, whereas subjects who reported 3 and 4 adverse events showed a significant increase in plasma cortisol following the nocebo challenge, subjects who reported 0 adverse events showed no changes.
Conclusions: These findings emphasize the importance of the doctor-patient communication in perceiving symptoms like pain, and suggest that those subjects with high prenatal maternal cortisol may be more sensitive to the effects of a negative communication in adulthood.
Keywords: adverse events; cortisol; hyperalgesia; nocebo; oxygen; placebo; prenatal.
© 2022 Giovanni Fioriti Editore s.r.l.
Conflict of interest statement
Competing interests: None.
Figures

References
-
- Abbassi-Ghanavati, M., Greer, L. G., & Cunningham, F.G. (2009). Pregnancy and laboratory studies: a reference table for clinicians. Obstetrics & Gynecology, 114, 1326-1331. - PubMed
-
- Benedetti, F., Amanzio, M., Casadio, C., Oliaro, A., & Maggi, G. (1997). Blockade of nocebo hyperalgesia by the cholecystokinin antagonist proglumide. Pain, 71, 135-140. - PubMed
-
- Benedetti, F., Amanzio, M., Giovanelli, F., Craigs-Brackhahn, K., & Shaibani, A. (2021). Hypothalamic-pituitary-adrenal activity in adverse events reporting after placebo administration. Clinical Pharmacology & Therapeutics, 110, 1349-1357. - PubMed
-
- Benedetti, F., Amanzio, M., Rosato, R., & Blanchard, C. (2011). Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nature Medicine, 17, 1228-1230. - PubMed
LinkOut - more resources
Full Text Sources
