Immunosuppressive Drugs in Liver Transplant: An Insight
- PMID: 36340316
- PMCID: PMC9630030
- DOI: 10.1016/j.jceh.2022.06.007
Immunosuppressive Drugs in Liver Transplant: An Insight
Abstract
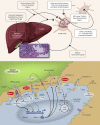
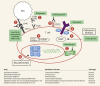
Liver transplantation (LT) is the standard of care for end-stage liver failure and hepatocellular carcinoma. Over the years, immunosuppression regimens have improved, resulting in enhanced graft and patient survival. At present, the side effects of immunosuppressive agents are a significant threat to post-LT quality of life and long-term outcome. The role of personalized immunosuppression is to reach a delicate balance between optimal immunosuppression and minimal side effects. Today, immunosuppression in LT is more of an art than a science. There are no validated markers for overimmunosuppression and underimmunosuppression, only a few drugs have therapeutic drug monitoring and immunosuppression regimens vary from center to center. The immunosuppressive agents are broadly classified into biological agents and pharmacological agents. Most regimens use multiple agents with different modes of action to reduce the dosage and minimize the toxicities. The calcineurin inhibitor (CNI)-related toxicities are reduced by antibody induction or using mTOR inhibitor/antimetabolites as CNI sparing or CNI minimization strategies. Post-liver transplant immunosuppression has an intensive phase in the first three months when alloreactivity is high, followed by a maintenance phase when immunosuppression minimization protocols are implemented. Over time some patients achieve "tolerance," defined as the successful stopping of immunosuppression with good graft function and no indication of rejection. Cell-based therapy using immune cells with tolerogenic potential is the future and may permit complete withdrawal of immunosuppressive agents.
Keywords: AMR, Antibody-mediated rejection; APCs, Antigen-presenting cells; ATG, Anti-thymocyte globulin; CNI, Calcineurin inhibitors; CsA, Cyclosporine A; EVR, Everolimus; IL-2R, Interleukin 2 Receptor; LT, Liver transplantation; MMF, Mycophenolate mofetil; MPA, Mycophenolic acid; SRL, Sirolimus; TAC, Tacrolimus; TCMR, T-cell-mediated rejection; antimetabolites; basiliximab; calcineurin inhibitors; cyclosporine; everolimus; immunosuppression; liver transplantation; mTORi, mammalian targets of rapamycin inhibitor; mycophenolate mofetil; tacrolimus.
© 2022 Indian National Association for Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Rana A., Ackah R.L., Webb G.J., et al. No gains in long-term survival after liver transplantation over the past three decades. Ann Surg. 2019 Jan;269:20–27. - PubMed
-
- Ojo A.O., Held P.J., Port F.K., et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003 Sep 4;349:931–940. - PubMed
-
- Charlton M., Levitsky J., Aqel B., et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018 May;102:727–743. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

