Psilocybin containing mushrooms: a rapidly developing biotechnology industry in the psychiatry, biomedical and nutraceutical fields
- PMID: 36340802
- PMCID: PMC9633885
- DOI: 10.1007/s13205-022-03355-4
Psilocybin containing mushrooms: a rapidly developing biotechnology industry in the psychiatry, biomedical and nutraceutical fields
Abstract
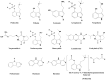
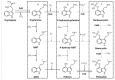
Humans have collected and used hallucinogenic mushrooms for ethnic medicinal, recreational, and religious purposes since before recorded history. Currently, the use of these mushrooms is illegal in most countries, but where their use is legal they are applied as self medication. Psilocybin and psilocin, two psychoactive alkaloids, are naturally synthesized by hallucinogenic mushrooms. The chemical structure of these compounds are similar to the neurotransmitter serotonin. Activation of this system by psilocybin and psilocin may produce temporary changes in the brain that induce hallucinations and feelings of euphoria. Adjustment of the serotonin system in this way can moderate symptoms of related mental disorders. This review summarizes relevant and current information regarding the discovery of hallucinogenic mushrooms and their contained psychoactive compounds, the events that lead to their criminalization and decriminilization, and the state of knowledge of psilocybin, psilocin, and derivatives. Last, research on the psychoactive properties of these mushrooms is placed in perspective to possible applications for human dysfunctions.
Keywords: Hallucinogenic medicine; Hallucinogenic mushrooms; Psilocin; Psilocybin; Serotonergic Neurotransmitter System.
© King Abdulaziz City for Science and Technology 2022, Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Agurell S, Blomkvist S, Catalfomo P. Biosynthesis of psilocybin in submerged culture of Psilocybe cubensis. 1. Incorporation of labelled tryptophan and tryptamine. Acta Pharm Suec. 1966;3:37–44. - PubMed
-
- Alam Mahmood Z. Bioactive alkaloids from fungi: Psilocybin. In: Ramawat KG, Merillon JM, editors. Natural products: phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Berlin Heidelberg: Springer-Verlag; 2013. pp. 523–552.
-
- Allen JW, Gartz J (2009) Magic mushrooms in some third world countries. Ethnomycological Journals 6, Seattle
Publication types
LinkOut - more resources
Full Text Sources

