N-[(E)-Quinolin-2-yl-methyl-idene]-1,2,4-triazol-4-amine hemihydrate
- PMID: 36340830
- PMCID: PMC9462182
- DOI: 10.1107/S2414314620001340
N-[(E)-Quinolin-2-yl-methyl-idene]-1,2,4-triazol-4-amine hemihydrate
Abstract
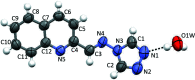
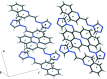
The title hemihydrate, C12H9N5·0.5H2O, was isolated from the condensation reaction of quinoline-2-carbaldehyde with 4-amino-4H-1,2,4-triazole. The Schiff base mol-ecule adopts an E configuration about the C=N bond and is approximately planar, with a dihedral angle between the quinoline ring system and the 1,2,4-triazole ring of 12.2 (1)°. In the crystal, one water mol-ecule bridges two Schiff base mol-ecules via O-H⋯N hydrogen bonds. The Schiff base mol-ecules are inter-connected by π-π stacking inter-actions [centroid-centroid distances of 3.7486 (7) and 3.9003 (7) Å] into columns along [10].
Keywords: Schiff base; crystal structure; green synthesis; intermolecular hydrogen bond; triazole.
© M. Shahri et al. 2020.
Figures

References
-
- Akin, S., Ayaloglu, H., Gultekin, E., Colak, A., Bekircan, O. & Akatin, M. Y. (2019). Bioorg. Chem. 83, 170–179. - PubMed
-
- Bhalgat, C. M., Irfan Ali, M., Ramesh, B. & Ramu, G. (2014). Arab. J. Chem, 7, 986–993.
-
- Bruker (2014). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Kołodziej, B., Morawiak, M., Schilf, W. & Kamieński, B. (2019). J. Mol. Struct. 1184, 207–218.
-
- Saadaoui, I., Krichen, F., Ben Salah, B., Ben Mansour, R., Miled, N., Bougatef, A. & Kossentini, M. (2019). J. Mol. Struct. 1180, 344–354.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
