Fecal microbiota transplantation in patients with post-infectious irritable bowel syndrome: A randomized, clinical trial
- PMID: 36341232
- PMCID: PMC9631772
- DOI: 10.3389/fmed.2022.994911
Fecal microbiota transplantation in patients with post-infectious irritable bowel syndrome: A randomized, clinical trial
Abstract
Introduction: Research in recent years has shown the potential benefits of fecal microbiota transplantation (FMT) for irritable bowel syndrome (IBS). Acute infectious gastroenteritis is a well-established risk factor for developing such forms of IBS as post-infectious IBS (PI-IBS). However, the effective use of FMT in patients with IP-IBS has not yet been clarified.
Aim: The study aimed to conduct a single-center, randomized clinical trial (RCT) to assess FMT's safety, clinical and microbiological efficacy in patients with PI-IBS.
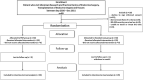
Materials and methods: Patients with PI-IBS were randomized into two groups: I (standard-care, n = 29) were prescribed basic therapy, namely a low FODMAP diet, as well as Otilonium Bromide (1 tablet TID) and a multi-strain probiotic (1 capsule BID) for 1 month; II (FMT group, n = 30), each patient with PI-IBS underwent a single FMT procedure with fresh material by colonoscopy. All patients underwent bacteriological examination of feces for quantitative and qualitative microbiota composition changes. The clinical efficacy of treatment was evaluated according to the dynamics of abdominal symptoms, measured using the IBS-SSS scale, fatigue reduction (FAS scale), and a change in the quality of life (IBS-QoL scale).
Results: FMT was associated with rapid onset of the effect, manifested in a significant difference between IBS-SSS points after 2 weeks of intervention (p < 0.001). In other time points (after 4 and 12 weeks) IBS-SSS did not differ significantly across both groups. Only after 3 months of treatment did their QoL exceed its initial level, as well value for 2 and 4 weeks, to a significant extent. The change in the ratio of the main microbial phenotypes in the form of an increase in the relative abundance of Firmicutes and Bacteroidetes was recorded in all patients after 4 weeks. It should be noted that these changes were significant but eventually normalized only in the group of PI-IBS patients who underwent FMT. No serious adverse reactions were noted.
Conclusion: This comparative study of the results of FMT use in patients with PI-IBS demonstrated its effectiveness compared to traditional pharmacotherapy, as well as a high degree of safety and good tolerability.
Keywords: diarrhea; dysbiosis; fecal microbiota transplantation; gut microbiota; post-infectious irritable bowel syndrome.
Copyright © 2022 Tkach, Dorofeyev, Kuzenko, Sulaieva, Falalyeyeva and Kobyliak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet (London, England). (2020) 396:1675–88. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

