Neutralizing antibodies from the rare convalescent donors elicited antibody-dependent enhancement of SARS-CoV-2 variants infection
- PMID: 36341247
- PMCID: PMC9627283
- DOI: 10.3389/fmed.2022.952697
Neutralizing antibodies from the rare convalescent donors elicited antibody-dependent enhancement of SARS-CoV-2 variants infection
Abstract
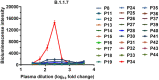
Currently, neutralizing antibody and vaccine strategies have been developed by targeting the SARS-CoV-2 strain identified during the early phase of the pandemic. Early studies showed that the ability of SARS-CoV-2 RBD or NTD antibodies to elicit infection enhancement in vivo is still controversial. There are growing concerns that the plasma and neutralizing antibodies from convalescent patients or people receiving vaccines mediate ADE of SARS-CoV-2 variants infections in immune cells. Here, we constructed engineered double-mutant variants containing an RBD mutation and D614G in the spike (S) protein and natural epidemic variants to gain insights into the correlation between the mutations in S proteins and the ADE activities and tested whether convalescent plasma and TOP10 neutralizing antibodies in our laboratory mediated the ADE effects of these SARS-CoV-2 variants. We found that one out of 29 convalescent plasma samples caused the ADE effect of pandemic variant B.1.1.7 and that the ADE effect of wild-type SARS-CoV-2 was not detected for any of these plasma samples. Only one antibody, 55A8, from the same batch of convalescent patients mediated the ADE effects of multiple SARS-CoV-2 variants in vitro, including six double-mutant variants and four epidemic variants, suggesting that ADE activities may be closely related to the antibody itself and the SARS-CoV-2 variants' S proteins. Moreover, the ADE activity of 55A8 depended on FcγRII on immune cells, and the introduction of LALA mutations at the Fc end of 55A8 eliminated the ADE effects in vitro, indicating that 55A8LALA may be a clinical drug used to prevent SARS-CoV-2 variants. Altogether, ADE may occur in rare convalescent patients or vaccinees with ADE-active antibodies who are then exposed to a SARS-CoV-2 variant. These data suggested that potential neutralizing antibodies may need to undergo ADE screening tests for SARS-CoV-2 variants, which should aid in the future design of effective antibody-based therapies.
Keywords: SARS-CoV-2 variants; antibody-dependent enhancement; infection-enhancing antibodies; neutralizing antibody; receptor-binding domain (RBD).
Copyright © 2022 Mu, Song, Hao, Luo, Wu, Wang, Han, Li, Hu, Li, Shen, Huang, Wang, Wang and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

