Transformable nanoparticles to bypass biological barriers in cancer treatment
- PMID: 36341301
- PMCID: PMC9595105
- DOI: 10.1039/d2na00485b
Transformable nanoparticles to bypass biological barriers in cancer treatment
Abstract
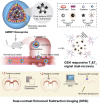
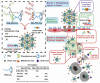
Nanomedicine based drug delivery platforms provide an interesting avenue to explore for the future of cancer treatment. Here we discuss the barriers for drug delivery in cancer therapeutics and how nanomaterials have been designed to bypass these blockades through stimuli responsive transformation in the most recent update. Nanomaterials that address the challenges of each step provide a promising solution for new cancer therapeutics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures








Similar articles
-
Nanoparticles with transformable physicochemical properties for overcoming biological barriers.Nanoscale. 2023 Aug 17;15(32):13202-13223. doi: 10.1039/d3nr01332d. Nanoscale. 2023. PMID: 37526946 Review.
-
Multistage Self-Assembled Nanomaterials for Cancer Immunotherapy.Molecules. 2023 Nov 24;28(23):7750. doi: 10.3390/molecules28237750. Molecules. 2023. PMID: 38067480 Free PMC article. Review.
-
Advances in intelligent-responsive nanocarriers for cancer therapy.Pharmacol Res. 2022 Apr;178:106184. doi: 10.1016/j.phrs.2022.106184. Epub 2022 Mar 14. Pharmacol Res. 2022. PMID: 35301111 Review.
-
Smart transformable nanomedicines for cancer therapy.Biomaterials. 2021 Apr;271:120737. doi: 10.1016/j.biomaterials.2021.120737. Epub 2021 Mar 2. Biomaterials. 2021. PMID: 33690103 Review.
-
Stimuli-responsive nanomaterials for therapeutic protein delivery.J Control Release. 2014 Nov 28;194:1-19. doi: 10.1016/j.jconrel.2014.08.015. Epub 2014 Aug 21. J Control Release. 2014. PMID: 25151983 Free PMC article. Review.
Cited by
-
Organic and inorganic nanomedicine for combination cancer therapies.Nanoscale Adv. 2023 Feb 23;5(6):1600-1610. doi: 10.1039/d3na00043e. eCollection 2023 Mar 14. Nanoscale Adv. 2023. PMID: 36926565 Free PMC article. Review.
-
Revolutionary NIR-activated silicon nanoparticles: precision-controlled release and targeted 3D cancer cell destruction.RSC Adv. 2025 Feb 14;15(7):4958-4969. doi: 10.1039/d4ra08889a. eCollection 2025 Feb 13. RSC Adv. 2025. PMID: 39957827 Free PMC article.

