Trends in industrialization and commercialization of IgY technology
- PMID: 36341353
- PMCID: PMC9630564
- DOI: 10.3389/fimmu.2022.991931
Trends in industrialization and commercialization of IgY technology
Abstract
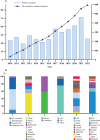
IgY technology refers to the strategic production process involved in generating avian immunoglobulin (IgY) against target antigens in a much more cost-effective manner with broad applications in the fields of diagnostics, prophylaxis, and therapeutics for both human and veterinary medicine. Over the past decade, promising progress in this research area has been evident from the steep increase in the number of registered manufacturing companies involved in the production of IgY products, the number of patents, and the notable number of clinical trials underway. Hence, it is crucial to conduct a prospective analysis of the commercialization and marketing potential of IgY-based commercial products for large-scale applications. This review revealed that the number of IgY patent applications increased steeply after 2010, with the highest of 77 patents filed in 2021. In addition, 73 industries are reportedly involved in marketing IgY products, out of which 27 were promoting biotherapeutics for human and veterinary medicine and 46 were in the diagnostic field. IgY antibodies are being used as primary and secondary antibodies, with approximately 3729 and 846 products, respectively. Biotherapeutic product consumption has notably increased as a food supplement and as a topical application in human and veterinary medicine, which are under different clinical phases of development to reach the market with around 80 and 56 products, respectively. In contrast, the number of IgY products as parenteral administrations and licensed drugs is not well developed given the lack of technical standards established for IgY registration and industrialization, as well as the restriction of the nature of polyclonal antibodies. However, recent ongoing research on functional IgY fragments indicates a promising area for IgY applications in the near future. Therefore, retrospective analysis with speculations is mandatory for IgY technology maturation toward industrialization and commercialization.
Keywords: IgY company; IgY patent; IgY product; commercialization; egg yolk antibody (IgY); industrialization.
Copyright © 2022 Yakhkeshi, Wu, Chelliappan and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Scientometric analysis and perspective of IgY technology study.Poult Sci. 2022 Apr;101(4):101713. doi: 10.1016/j.psj.2022.101713. Epub 2022 Jan 10. Poult Sci. 2022. PMID: 35150941 Free PMC article.
-
IgY-technology (egg yolk antibodies) in human medicine: A review of patents and clinical trials.Int Immunopharmacol. 2020 Apr;81:106269. doi: 10.1016/j.intimp.2020.106269. Epub 2020 Feb 6. Int Immunopharmacol. 2020. PMID: 32036273 Review.
-
IgY technology: Methods for developing and evaluating avian immunoglobulins for the in vitro detection of biomolecules.World J Methodol. 2021 Sep 20;11(5):243-262. doi: 10.5662/wjm.v11.i5.243. eCollection 2021 Sep 20. World J Methodol. 2021. PMID: 34631482 Free PMC article. Review.
-
Production and Evaluation of Chicken Egg Yolk Immunoglobulin (IgY) against Human and Simian Rotaviruses.Viruses. 2022 Sep 9;14(9):1995. doi: 10.3390/v14091995. Viruses. 2022. PMID: 36146801 Free PMC article.
-
Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine.Altern Lab Anim. 2005 Apr;33(2):129-54. doi: 10.1177/026119290503300208. Altern Lab Anim. 2005. PMID: 16180988 Review.
Cited by
-
A novel and quick egg yolk immunoglobulin y antibody extraction method leveraging the protein liquid-liquid phase separation principle.Poult Sci. 2025 Feb;104(2):104804. doi: 10.1016/j.psj.2025.104804. Epub 2025 Jan 11. Poult Sci. 2025. PMID: 39813868 Free PMC article.
-
Comparison of immunoglobulin Y antibody production in new and spent laying hens.Vet World. 2024 Sep;17(9):2177-2184. doi: 10.14202/vetworld.2024.2177-2184. Epub 2024 Sep 28. Vet World. 2024. PMID: 39507799 Free PMC article.
-
A novel antibody treatment reduces deformed wing virus loads in the western honey bee (Apis mellifera).mSphere. 2024 Nov 21;9(11):e0049724. doi: 10.1128/msphere.00497-24. Epub 2024 Oct 30. mSphere. 2024. PMID: 39475320 Free PMC article.
-
Monoclonal IgY antibodies: advancements and limitations for immunodiagnosis and immunotherapy applications.Ther Adv Vaccines Immunother. 2024 Jul 25;12:25151355241264520. doi: 10.1177/25151355241264520. eCollection 2024. Ther Adv Vaccines Immunother. 2024. PMID: 39071998 Free PMC article. Review.
-
Development of an ostrich-derived single-chain variable fragment (scFv) against PTPRN extracellular domain.Sci Rep. 2024 Feb 14;14(1):3689. doi: 10.1038/s41598-024-53386-5. Sci Rep. 2024. PMID: 38355744 Free PMC article.
References
-
- Zhang ZX, Vieira-Pires RS, Morgan PM, Schade R. Chapter 17. (IgY industries and markets). in: IgY-technology: Production and application of egg yolk antibodies. Springer Sci (2021) 1:279–308. doi: 10.1007/978-3-030-72688-1 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

