Abrogation of neutrophil inflammatory pathways and potential reduction of neutrophil-related factors in COVID-19 by intravenous immunoglobulin
- PMID: 36341409
- PMCID: PMC9632428
- DOI: 10.3389/fimmu.2022.993720
Abrogation of neutrophil inflammatory pathways and potential reduction of neutrophil-related factors in COVID-19 by intravenous immunoglobulin
Abstract
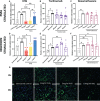
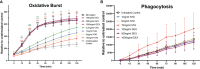
Pathogenesis of lung injury in COVID-19 is not completely understood, leaving gaps in understanding how current treatments modulate the course of COVID-19. Neutrophil numbers and activation state in circulation have been found to correlate with COVID-19 severity, and neutrophil extracellular traps (NETs) have been found in the lung parenchyma of patients with acute respiratory distress syndrome (ARDS) in COVID-19. Targeting the pro-inflammatory functions of neutrophils may diminish lung injury in COVID-19 and ARDS. Neutrophils were isolated from peripheral blood of healthy donors, treated ex vivo with dexamethasone, tocilizumab and intravenous immunoglobulin (IVIG) and NET formation, oxidative burst, and phagocytosis were assessed. Plasma from critically ill COVID-19 patients before and after clinical treatment with IVIG and from healthy donors was assessed for neutrophil activation-related proteins. While dexamethasone and tocilizumab did not affect PMA- and nigericin-induced NET production ex vivo, IVIG induced a dose-dependent abrogation of NET production in both activation models. IVIG also reduced PMA-elicited reactive oxygen species production, but did not alter phagocytosis. COVID-19 patients were found to have elevated levels of cell-free DNA, neutrophil elastase and IL-8 as compared to healthy controls. Levels of both cell-free DNA and neutrophil elastase were lower 5 days after 4 days of daily treatment with IVIG. The lack of impact of dexamethasone or tocilizumab on these neutrophil functions suggests that these therapeutic agents may not act through suppression of neutrophil functions, indicating that the door might still be open for the addition of a neutrophil modulator to the COVID-19 therapeutic repertoire.
Keywords: COVID-19; NETosis; corticosteroids; dexamethasone; intravenous immunoglobulin (IVIG); neutrophils; oxidative burst; tocilizumab.
Copyright © 2022 Masso-Silva, Sakoulas, Olay, Groysberg, Geriak, Nizet, Crotty Alexander and Meier.
Conflict of interest statement
Author GS has received consulting and research fees from Octapharma USA (Hoboken, NJ). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures