Helicobacter pylori-infected human neutrophils exhibit impaired chemotaxis and a uropod retraction defect
- PMID: 36341418
- PMCID: PMC9630475
- DOI: 10.3389/fimmu.2022.1038349
Helicobacter pylori-infected human neutrophils exhibit impaired chemotaxis and a uropod retraction defect
Abstract
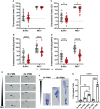
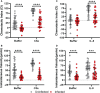
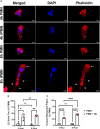
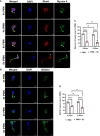
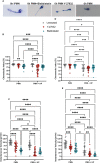
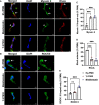
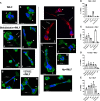
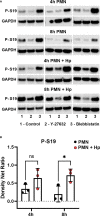
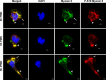
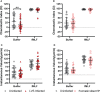
Helicobacter pylori is a major human pathogen that colonizes the gastric mucosa and plays a causative role in development of peptic ulcers and gastric cancer. Neutrophils are heavily infected with this organism in vivo and play a prominent role in tissue destruction and disease. Recently, we demonstrated that H. pylori exploits neutrophil plasticity as part of its virulence strategy eliciting N1-like subtype differentiation that is notable for profound nuclear hypersegmentation. We undertook this study to test the hypothesis that hypersegmentation may enhance neutrophil migratory capacity. However, EZ-TAXIScan™ video imaging revealed a previously unappreciated and progressive chemotaxis defect that was apparent prior to hypersegmentation onset. Cell speed and directionality were significantly impaired to fMLF as well as C5a and IL-8. Infected cells oriented normally in chemotactic gradients, but speed and direction were impaired because of a uropod retraction defect that led to cell elongation, nuclear lobe trapping in the contracted rear and progressive narrowing of the leading edge. In contrast, chemotactic receptor abundance, adhesion, phagocytosis and other aspects of cell function were unchanged. At the molecular level, H. pylori phenocopied the effects of Blebbistatin as indicated by aberrant accumulation of F-actin and actin spikes at the uropod together with enhanced ROCKII-mediated phosphorylation of myosin IIA regulatory light chains at S19. At the same time, RhoA and ROCKII disappeared from the cell rear and accumulated at the leading edge whereas myosin IIA was enriched at both cell poles. These data suggest that H. pylori inhibits the dynamic changes in myosin IIA contractility and front-to-back polarity that are essential for chemotaxis. Taken together, our data advance understanding of PMN plasticity and H. pylori pathogenesis.
Keywords: H. pylori; ROCK; RhoA; actin; chemotaxis; myosin II; neutrophils; uropod.
Copyright © 2022 Prichard, Khuu, Whitmore, Irimia and Allen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cutting Edge: Helicobacter pylori Induces Nuclear Hypersegmentation and Subtype Differentiation of Human Neutrophils In Vitro.J Immunol. 2017 Mar 1;198(5):1793-1797. doi: 10.4049/jimmunol.1601292. Epub 2017 Feb 1. J Immunol. 2017. PMID: 28148734 Free PMC article.
-
Filamin-A regulates neutrophil uropod retraction through RhoA during chemotaxis.PLoS One. 2013 Oct 25;8(10):e79009. doi: 10.1371/journal.pone.0079009. eCollection 2013. PLoS One. 2013. PMID: 24205360 Free PMC article.
-
Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration.J Cell Sci. 2000 Apr;113 ( Pt 7):1287-98. doi: 10.1242/jcs.113.7.1287. J Cell Sci. 2000. PMID: 10704379
-
Phagocytosis and persistence of Helicobacter pylori.Cell Microbiol. 2007 Apr;9(4):817-28. doi: 10.1111/j.1462-5822.2007.00906.x. Epub 2007 Mar 8. Cell Microbiol. 2007. PMID: 17346311 Review.
-
The neutrophil-activating protein of Helicobacter pylori.Int J Med Microbiol. 2002 Feb;291(6-7):545-50. doi: 10.1078/1438-4221-00165. Int J Med Microbiol. 2002. PMID: 11890556 Review.
Cited by
-
Immune Biology and Persistence of Helicobacter pylori in Gastric Diseases.Curr Top Microbiol Immunol. 2023;444:83-115. doi: 10.1007/978-3-031-47331-9_4. Curr Top Microbiol Immunol. 2023. PMID: 38231216
-
Helicobacter pylori and gastric cancer: mechanisms and new perspectives.J Hematol Oncol. 2025 Jan 23;18(1):10. doi: 10.1186/s13045-024-01654-2. J Hematol Oncol. 2025. PMID: 39849657 Free PMC article. Review.
-
Decoding the Ability of Helicobacter pylori to Evade Immune Recognition and Cause Disease.Cell Mol Gastroenterol Hepatol. 2025;19(5):101470. doi: 10.1016/j.jcmgh.2025.101470. Epub 2025 Jan 30. Cell Mol Gastroenterol Hepatol. 2025. PMID: 39889829 Free PMC article. Review.
-
Multi-omics analysis of Helicobacter pylori-associated gastric cancer identifies hub genes as a novel therapeutic biomarker.Brief Bioinform. 2025 May 1;26(3):bbaf241. doi: 10.1093/bib/bbaf241. Brief Bioinform. 2025. PMID: 40445003 Free PMC article.
-
Effects of the pan-caspase inhibitor Q-VD-OPh on human neutrophil lifespan and function.PLoS One. 2025 Jan 7;20(1):e0316912. doi: 10.1371/journal.pone.0316912. eCollection 2025. PLoS One. 2025. PMID: 39775346 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

