Managing the TME to improve the efficacy of cancer therapy
- PMID: 36341428
- PMCID: PMC9630343
- DOI: 10.3389/fimmu.2022.954992
Managing the TME to improve the efficacy of cancer therapy
Abstract
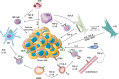
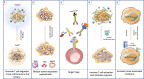
The tumor microenvironment (TME) influences tumor growth, metastatic spread and response to treatment. Often immunosuppression, mediated by the TME, impairs a beneficial response. The complexity of the tumor composition challenges our abilities to design new and more effective therapies. Going forward we will need to 'manage' the content and or functionality of the TME to improve treatment outcomes. Currently, several different kinds of treatments are available to patients with cancer: there are the traditional approaches of chemotherapy, radiation and surgery; there are targeted agents that inhibit kinases associated with oncogenic pathways; there are monoclonal antibodies that target surface antigens often delivering toxic payloads or cells and finally there are antibodies and biologics that seek to overcome the immunosuppression caused by elements within the TME. How each of these therapies interact with the TME is currently under intense and widespread investigation. In this review we describe how the TME and its immunosuppressive components can influence both tumor progression and response to treatment focusing on three particular tumor types, classic Hodgkin Lymphoma (cHL), Pancreatic Ductal Adenocarcinoma (PDAC) and Glioblastoma Multiforme (GBM). And, finally, we offer five approaches to manipulate or manage the TME to improve outcomes for cancer patients.
Keywords: GBM - glioblastoma multiforme; Hodgkin (cHL); PDAC - pancreatic ductal adenocarcinoma; TME (tumor microenvironment); anti-cancer therapy; immunosuppression; immunotherapy combined therapy.
Copyright © 2022 Bilotta, Antignani and Fitzgerald.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov (2021) 11(4):933–59. doi: 10.1158/2159-8290.CD-20-1808 - DOI - PubMed
-
- Aoki T, Chong LC, Takata K K, Milne K, Hav M, Colombo A, et al. . Single-cell transcriptome analysis reveals disease-defining T-cell subsets in the tumor microenvironment of classic Hodgkin lymphoma. Cancer Discov (2020) 10(3):406–21. doi: 10.1158/2159-8290.CD-19-0680 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

