The role of cuproptosis-related gene in the classification and prognosis of melanoma
- PMID: 36341437
- PMCID: PMC9632664
- DOI: 10.3389/fimmu.2022.986214
The role of cuproptosis-related gene in the classification and prognosis of melanoma
Abstract
Background: Melanoma, as one of the most aggressive and malignant cancers, ranks first in the lethality rate of skin cancers. Cuproptosis has been shown to paly a role in tumorigenesis, However, the role of cuproptosis in melanoma metastasis are not clear. Studying the correlation beteen the molecular subtypes of cuproptosis-related genes (CRGs) and metastasis of melanoma may provide some guidance for the prognosis of melanoma.
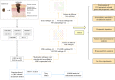
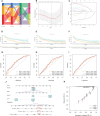
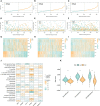
Methods: We collected 1085 melanoma samples in The Cancer Genome Atlas(TCGA) and Gene Expression Omnibus(GEO) databases, constructed CRGs molecular subtypes and gene subtypes according to clinical characteristics, and investigated the role of CRGs in melanoma metastasis. We randomly divide the samples into train set and validation set according to the ratio of 1:1. A prognostic model was constructed using data from the train set and then validated on the validation set. We performed tumor microenvironment analysis and drug sensitivity analyses for high and low risk groups based on the outcome of the prognostic model risk score. Finally, we established a metastatic model of melanoma.
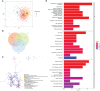
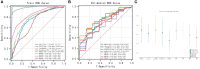
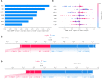
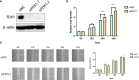
Results: According to the expression levels of 12 cuproptosis-related genes, we obtained three subtypes of A1, B1, and C1. Among them, C1 subtype had the best survival outcome. Based on the differentially expressed genes shared by A1, B1, and C1 genotypes, we obtained the results of three gene subtypes of A2, B2, and C2. Among them, the B2 group had the best survival outcome. Then, we constructed a prognostic model consisting of 6 key variable genes, which could more accurately predict the 1-, 3-, and 5-year overall survival rates of melanoma patients. Besides, 98 drugs were screened out. Finally, we explored the role of cuproptosis-related genes in melanoma metastasis and established a metastasis model using seven key genes.
Conclusions: In conclusion, CRGs play a role in the metastasis and prognosis of melanoma, and also provide new insights into the underlying pathogenesis of melanoma.
Keywords: cuproptosis; machine learning; melanoma; metastasis model; prognostic model; subtype.
Copyright © 2022 Liu, Liu, Li, Luo and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Identification of cuproptosis-related subtypes, construction of a prognosis model, and tumor microenvironment landscape in gastric cancer.Front Immunol. 2022 Nov 21;13:1056932. doi: 10.3389/fimmu.2022.1056932. eCollection 2022. Front Immunol. 2022. PMID: 36479114 Free PMC article.
-
Relationships of Cuproptosis-Related Genes With Clinical Outcomes and the Tumour Immune Microenvironment in Hepatocellular Carcinoma.Pathol Oncol Res. 2022 Sep 21;28:1610558. doi: 10.3389/pore.2022.1610558. eCollection 2022. Pathol Oncol Res. 2022. PMID: 36213162 Free PMC article.
-
Cuproptosis-related risk score predicts prognosis and characterizes the tumor microenvironment in colon adenocarcinoma.Front Oncol. 2023 Jun 2;13:1152681. doi: 10.3389/fonc.2023.1152681. eCollection 2023. Front Oncol. 2023. PMID: 37333810 Free PMC article.
-
Identification of a novel cuproptosis-related gene signature and integrative analyses in patients with lower-grade gliomas.Front Immunol. 2022 Aug 15;13:933973. doi: 10.3389/fimmu.2022.933973. eCollection 2022. Front Immunol. 2022. PMID: 36045691 Free PMC article. Review.
-
The Genomic Landscape of Melanoma and Its Therapeutic Implications.Genes (Basel). 2023 Apr 29;14(5):1021. doi: 10.3390/genes14051021. Genes (Basel). 2023. PMID: 37239381 Free PMC article. Review.
Cited by
-
Cell Death Modalities in Therapy of Melanoma.Int J Mol Sci. 2025 Apr 8;26(8):3475. doi: 10.3390/ijms26083475. Int J Mol Sci. 2025. PMID: 40331942 Free PMC article. Review.
-
Integrated analysis of single-cell RNA-seq and chipset data unravels PANoptosis-related genes in sepsis.Front Immunol. 2024 Jan 3;14:1247131. doi: 10.3389/fimmu.2023.1247131. eCollection 2023. Front Immunol. 2024. PMID: 38239341 Free PMC article.
-
Cuproptosis: molecular mechanisms, cancer prognosis, and therapeutic applications.J Transl Med. 2025 Jan 22;23(1):104. doi: 10.1186/s12967-025-06121-1. J Transl Med. 2025. PMID: 39844182 Free PMC article. Review.
-
CDKN2A, a key gene in copper-induced cell death model, influencing melanoma invasion and apoptosis.Discov Oncol. 2025 Feb 27;16(1):246. doi: 10.1007/s12672-025-01992-8. Discov Oncol. 2025. PMID: 40014167 Free PMC article.
-
Role of hippo pathway and cuproptosis-related genes in immune infiltration and prognosis of skin cutaneous melanoma.Front Pharmacol. 2024 Mar 7;15:1344755. doi: 10.3389/fphar.2024.1344755. eCollection 2024. Front Pharmacol. 2024. PMID: 38515849 Free PMC article.
References
-
- Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 world health organization classification of cutaneous, mucosal, and uveal melanoma: Detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med (2020) 144:500–22. doi: 10.5858/arpa.2019-0561-RA - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

