Immune defenses of the mammary gland epithelium of dairy ruminants
- PMID: 36341445
- PMCID: PMC9634088
- DOI: 10.3389/fimmu.2022.1031785
Immune defenses of the mammary gland epithelium of dairy ruminants
Abstract
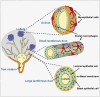
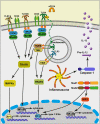
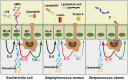
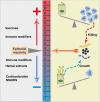
The epithelium of the mammary gland (MG) fulfills three major functions: nutrition of progeny, transfer of immunity from mother to newborn, and its own defense against infection. The defense function of the epithelium requires the cooperation of mammary epithelial cells (MECs) with intraepithelial leucocytes, macrophages, DCs, and resident lymphocytes. The MG is characterized by the secretion of a large amount of a nutrient liquid in which certain bacteria can proliferate and reach a considerable bacterial load, which has conditioned how the udder reacts against bacterial invasions. This review presents how the mammary epithelium perceives bacteria, and how it responds to the main bacterial genera associated with mastitis. MECs are able to detect the presence of actively multiplying bacteria in the lumen of the gland: they express pattern recognition receptors (PRRs) that recognize microbe-associated molecular patterns (MAMPs) released by the growing bacteria. Interactions with intraepithelial leucocytes fine-tune MECs responses. Following the onset of inflammation, new interactions are established with lymphocytes and neutrophils recruited from the blood. The mammary epithelium also identifies and responds to antigens, which supposes an antigen-presenting capacity. Its responses can be manipulated with drugs, plant extracts, probiotics, and immune modifiers, in order to increase its defense capacities or reduce the damage related to inflammation. Numerous studies have established that the mammary epithelium is a genuine effector of both innate and adaptive immunity. However, knowledge gaps remain and newly available tools offer the prospect of exciting research to unravel and exploit the multiple capacities of this particular epithelium.
Keywords: MAMPs; PRR; bacteria; epithelial cells; innate immunity; macrophages; mastitis.
Copyright © 2022 Rainard, Gilbert and Germon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Innate immunity of the bovine mammary gland.Vet Res. 2006 May-Jun;37(3):369-400. doi: 10.1051/vetres:2006007. Epub 2006 Feb 23. Vet Res. 2006. PMID: 16611554 Review.
-
Immune defences of the mammary gland in dairy ruminants.Reprod Domest Anim. 2023 Sep;58 Suppl 2:4-14. doi: 10.1111/rda.14372. Epub 2023 Jul 12. Reprod Domest Anim. 2023. PMID: 37133304 Review.
-
Repertoire of Escherichia coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells.Vet Res. 2012 Feb 13;43(1):14. doi: 10.1186/1297-9716-43-14. Vet Res. 2012. PMID: 22330199 Free PMC article.
-
Mobilization of neutrophils and defense of the bovine mammary gland.Reprod Nutr Dev. 2003 Sep-Oct;43(5):439-57. doi: 10.1051/rnd:2003031. Reprod Nutr Dev. 2003. PMID: 15005373 Review.
-
Acute phase protein expressions in secretory and cistern lining epithelium tissues of the dairy cattle mammary gland during chronic mastitis caused by staphylococci.BMC Vet Res. 2020 Aug 31;16(1):320. doi: 10.1186/s12917-020-02544-8. BMC Vet Res. 2020. PMID: 32867772 Free PMC article.
Cited by
-
Punch-excised explants of bovine mammary gland to model early immune response to infection.J Anim Sci Biotechnol. 2023 Jul 7;14(1):100. doi: 10.1186/s40104-023-00899-0. J Anim Sci Biotechnol. 2023. PMID: 37420291 Free PMC article.
-
Different Immune Control of Gram-Positive and Gram-Negative Mammary Infections in Dairy Cows.Vet Sci. 2024 Apr 6;11(4):166. doi: 10.3390/vetsci11040166. Vet Sci. 2024. PMID: 38668433 Free PMC article.
-
Differential expression of TLR and CXCR genes in mammary HC11 cells challenged with Bacillus cereus and Bacillus subtilis: Implications for mastitis resistance.Vet World. 2025 Apr;18(4):1014-1024. doi: 10.14202/vetworld.2025.1014-1024. Epub 2025 Apr 25. Vet World. 2025. PMID: 40453956 Free PMC article.
-
In vitro model of human mammary gland microbial colonization (MAGIC) demonstrates distinctive cytokine response to imbalanced human milk microbiota.Microbiol Spectr. 2024 Mar 5;12(3):e0236923. doi: 10.1128/spectrum.02369-23. Epub 2024 Jan 30. Microbiol Spectr. 2024. PMID: 38289112 Free PMC article.
-
Bovine neutrophils stimulated with Streptococcus uberis induce neutrophil extracellular traps, and cause cytotoxicity and transcriptional upregulation of inflammatory cytokine genes in bovine mammary epithelial cells.J Vet Med Sci. 2024 Feb 8;86(2):141-149. doi: 10.1292/jvms.23-0302. Epub 2023 Dec 18. J Vet Med Sci. 2024. PMID: 38104974 Free PMC article.
References
-
- Kornalijnslijper JE, Daemen AJ, van Werven T, Niewold TA, Rutten VP, Noordhuizen-Stassen EN. Bacterial growth during the early phase of infection determines the severity of experimental Escherichia coli mastitis in dairy cows. Vet Microbiol (2004) 101(3):177–86. doi: 10.1016/j.vetmic.2004.04.005 - DOI - PubMed
-
- Kierszenbaum AL, Tres LL. Histology and cell biology. an introduction to pathology. 3rd Edition ed. Philadelphia, PA, USA: Elsevier Saunders; (2012). 701 p.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

